crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
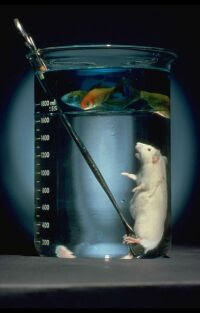
Liquid breathing: Perfluorocarbons!
Perfluorocarbons are compounds made of carbon and fluorine, they are usually liquid at room temperature and are highly inert. They also have the
interesting property of readily dissolving oxygen, because of this property and their inert nature they can be used for liquid breathing. Liquid
breathing is when a subjects lungs are partially or completely filled with oxygenated perfluorocarbons such as perfluorohexane.
Experiments have been carried out on sheep, rats, mice and humans. The potential benefits and uses include aiding premature infants with breathing
difficulties and helping burn victims. They also have the potential to be used for ultra deep sea diving as the perfluorocarbon will not be compressed
even at extreme depth.
3M makes a product called "Fluorinert" which is used for electronic applications, it is a mixture of several perfluorocarbon compounds though it's
exact formula is likely propriety it has been used successfully in lab experiments on animals.
Sigma sells perfluorohexane for $60 for 15ml while Fluorinert can be purchased at a rate of about $150 for 200ml.
I don't intend to experiment with these compounds because of the cost and the fact that mice may experience death as a result of lung trauma after
liquid breathing. I just thought this was cool and wanted to share it with you.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That has been known for something like 20 years, at least. I have also heard of the stuff being used as a blood substitute.
However, those prices quoted by Sigma for perfluorocarbons of the right molecular weight and oxygen solubility mean that obtaining enough to
comfortably submerge a large animal such as an human would be quite expensive, and besides oxygen would have to be continually introduced to the
liquid and at the same time the CO2 waste product somehow removed. They are fairly costly because of the processes that have to be employed to replace
H atoms in hydrocarbons with F atoms without either breaking C-C bonds or adding across C=C double bonds: either catalytically using CoF3 which gives
up an F atom to form CoF2 which can be fluorinated back to CoF3, or else by electrolysis of a polar hydrocarbon-derived substrate dissolved in HF, see
http://en.wikipedia.org/wiki/Fluorocarbon . For all practical purposes, direct fluorination of hydrocarbons results only in CF4, and in some
circumstances C2F6.
The more viscous perfluorocarbons are mainly used, as a suspension in hydrocarbon lubricating oil, for extra-long-life lubricants in engines, aided by
the fact that they coat the inside surfaces which greatly reduces wear.
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
This is so cool.
One of my diving instructors told me about this stuff years ago.
I haven't really looked for any references at the times i remembered the conversation.
As a diver i find this very cool and maybe in the future it will be possible for humans to actually use this for diving.
The instructor told me that the amount that would be necessary compared to the conventional bottle would be a lot smaller.
This opens possibilities beyond imagination.
Thank you very much for this post.
What a fine day for chemistry this is.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
This is an interesting find! It means, as User said, that SCUBA gear could, hypothetically, be made far lighter and much more portable.
It also could pose some interesting possibilities in the future, perhaps for incarceration of criminals serving a life term - kept sedated and fed
via drip and suspended in a tank of this stuff... But that is just the first idea to pop up in my deranged mind at this late hour of night.
Still... VERY interesting. Now... How can we use it for our ends?
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Perfluorocarbons have hydrogen replaced with fluorine and are colorless inert
fluids that are passive carriers of oxygen. Solubility at S.T.P. up to 45 ml per
100 ml varies linearly with the gas pressure.
http://www.economicexpert.com/a/Liquid:breathing.html
http://en.wikipedia.org/wiki/Liquid_breathing
http://biomed.brown.edu/Courses/BI108/BI108_2005_Groups/10/w...
Google this search -> perfluorocarbon oxygen
_______________________________________________
http://www.fluoros.co.uk/data/medical_applications.php
3M Product Information Sheet , Data Sheet ,
http://solutions.3m.com/wps/portal/3M/en_WW/electronics/home...
http://solutions.3m.com/wps/portal/3M/en_WW/electronics/home...
_________________________________________________________
Blood substitutes Artificial oxygen carriers: perfluorocarbon emulsions
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC137239/pdf/cc364...
Or else from here _
http://www.biomedcentral.com/content/pdf/cc364.pdf
Partial Liquid Ventilation with Perfluorocarbon in Acute Lung Injury
http://ajrcmb.atsjournals.org/cgi/reprint/22/4/441
Solubility of Oxygen in Liquid Perfluorocarbons
http://symp15.nist.gov/pdf/p193.pdf
Patent US 5080885 - Brominated Perfluorocarbon Emulsions For
Internal Animal Use For Contrast Enhancement and Oxygen Transport
http://free.patentfetcher.com/GetPatentPDF.php?f=Pats/US/50/...
.
Attachment: Solubility of oxygen in substituted perfluorocarbons.pdf (137kB)
This file has been downloaded 994 times
Attachment: Perfluoro carbons Compounds used as Oxygen Carriers.pdf (280kB)
This file has been downloaded 4467 times
|
|
|
astroturf
Harmless

Posts: 6
Registered: 15-10-2009
Location: Melbourne, Australia.
Member Is Offline
Mood: No Mood
|
|
Haven't you seen The Abyss?
[Edited on 16-1-2010 by astroturf]
|
|
|