fresher_007
Harmless

Posts: 10
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
tetra butyl ammonium bromide
hi every body,
i have tri butyl amine , butyl bromide.........
can anybody suggest me by which route i should make tetrabutylammonium bromide.............
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Mix them and set aside for a couple of months....
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
It would have to be done under conditions that promote the ionization of the butyl bromide to the butyl carbocation and Br-, which would usually be a
polar aprotic solvent. The butyl carbocation then adds by electrophilic addition to the unutilized electron pair of the tertiary amine N, to form
[N(C4H9)4]+ . Formation of the carbocation would be faster with tert-butyl bromide, and to a lesser extent sec-butyl bromide, than with
n-butyl-1-bromide.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
JohnWW, what you say makes no sense. This reaction (the so called Menshutkin reaction) is a typical SN2 substitution and thus no carbocations are
involved at all. Besides, simple primary alkyl carbocations do not really exist or are utmost transient species (they rearrange during the formation
via rapid hydride shifts anyway). That is why SN1 substitutions with such activated primary alkyls generally don't work at all or give only
rearrangement products under forcing conditions (the only exceptions are R-CH2X where the R group has a strong enough stabilizing effect).
Fresher_007, is there a reason you don't want to look up the literature for examples? This is already the second time you open threads asking about
trivial reactions with plenty of literature examples and not providing a single reference. This forum is not a free service for literature search. You
are supposed to show that you invested at least some minimum effort before asking others to do your work.
PS: This goes in the Beginnings section.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
24-2-2010 at 14:35 |
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I disagree. A carbocation MUST be formed, one way or another, in order for a quaternary ammonium salt NR4+ to be formed. There is no way around that.
And if it has to be formed using a tertiary amine and an alkyl halide, ionization of the latter MUST occur, and indeed has been demonstrated to occur,
although usually to only a slight extent (unless a resonance-stabilized aromatic carbocation is formed) under suitable condition. That slight
ionization, in equilibrium, is enough for the reaction to occur, by the equilibrium continually shifting as the carbocation fraction reacts.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
JohnWW, this is very basic organic chemistry so I will not go into details which you can read in school books. I will just try to show you why what
you say is so illogic and counterintuitive. Basically, a carbocation MUST not form or else the product is not tetrabutylammonium bromide. You wonder
why? Now, just consider what would be a product of a hypothetical reaction between n-butyl bromide and tributylamine in methanol as solvent if the
reaction would proceed via a SN1 mechanism as you claim? If a butyl carbocation would be an intermediate, then the main products should be the methyl
s-butyl ether and 1-butene, but we know from experience that tetrabutylammonium bromide forms as the only noteworthy product. Therefore the reaction
can only proceed via a simple SN2 mechanism and no carbocation must ever be involved.
See the attached paper for experimental proof. SN1 substitutions generally require acidic media and nucleophiles of low basicity. Only few proceed
with basic nucleophiles and without acid catalysis (one such example is the reaction of trityl chloride with amines in aprotic solvents). Obviously,
tributylamine is a basic nucleophile.
Attachment: The solvent effect on nucleophilicity in the Menschutkin reaction.pdf (458kB)
This file has been downloaded 1167 times
|
|
|
fresher_007
Harmless

Posts: 10
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
tetra ethyl ammonium bromide preparation.....
i took triethyl amine & IPA.... and perged ethyl bromide gas.... but it took me 4 hrs to complete 20 % reaction.....
is this a right route ?????
if any other route please suggest me .........
|
|
|
Nicodem
|
Threads Merged
29-3-2010 at 00:53 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Fresher_007, instead of opening new threads for every quaternary ammonium salt you want to prepare (and again without references) check the replies in
the old one first. I merged the threads for your convenience.
Your question is formulated in a silly way. You ask us to analyse what is wrong with your procedure, but you do not write the experimental. Are we to
do guesswork? And do you really want to trust an answer based on guesswork? Now, write down the full experimental procedure. Then explain how you
measured the amount of EtBr absorbed, given you added it in the form of gas. Explain why you added gases EtBr instead of liquid. Describe which method
you used to measured the conversion. And so on...
While you are at it, explain also what literature you checked and provide references. Obviously, the only way you will get a good answer is for those
who are willing to answer to get acquainted with what is known about the pertaining reaction.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
I swear nicodem are you sure you did'nt co-author "advanced organic chemistry" j. march. ???
I ask because reading your posts is just like that.
I have to read, then, reread then think, then, read again; before i fully grasp what's being said.
[Edited on 2-4-2010 by jon]
|
|
|
fresher_007
Harmless

Posts: 10
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
i took
part A :
tri ethyl amine : 1010 gm
IPA : 600 gm
part B :
Hbr 35 % : 3000 gm
ethanol : 550 gm
sulphuric acid : 1600 gm
purged ethyl bromide gas from part B to the solution of part A...
reaction completed only 20 %........
plz give me any other route..... available.......
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Here is a web-page about the successive replacement of the Hs in NH3 by alkyl groups, by reaction of NH3 with an alkyl halide, successively forming a
primary, secondary, and tertiary amine; and then the tertiary amine reacting with the alkyl halide (usually the chloride or bromide) to form a
quaternary ammonium salt:
http://www.chemguide.co.uk/mechanisms/nucsub/amines.html
The nucleophilic substitution mechanism propounded on this page, for successive replacement of the Hs in NH3 by alkyl groups from a polar alkyl halide
to form the primary, secondary, and tertiary amines, is clearly correct. This is because the substituted Hs can be easily removed as H+ by other NH3
molecules to form ammonium chloride or bromide.
HOWEVER, this cannot be said for the final formation of a quaternary ammonium salt from the tertiary amine by reaction with another alkyl halide
molecule, near the bottom of the page, simply because there is NOTHING on the tertiary amine to be nucleophilically "substituted" for, meaning that
this final step CANNOT be a genuine "nucleophilic substitution". This part of the page reads as follows:
"A quaternary ammonium salt is an ammonium salt (for example, NH4+ Br-) in which all the hydrogens have been replaced by an alkyl group - for
example, (CH3CH2)4N+ Br-.
The mechanism
The tertiary amine still has an active lone pair on the nitrogen and, once again, that can attack the d+ carbon in the bromoethane.
But this time there is nowhere else for the reaction to go. There is no longer a hydrogen atom on the nitrogen that an ammonia molecule could remove,
and so the reaction finally comes to an end.
The product is a salt called tetraethylammonium bromide, (CH3CH2)4N+ Br-. "
This does not mention nucleophilic substitution, but it still implies nucleophilic "attack" by the lone electron pair on the N atom. However, in my
view, this is unsatisfactory, because it implies the formation of a reactive intermediate in which the halide C atom has a coordination number of 5
(at least 3 of which are to bulky atoms other than Hs), which is inconsistent with the available number of sp³ hybrid orbitals. This is why I
consider that an alternative mechanism, invoking the slight degree of formation from an alkyl (or aryl) halide of a carbocation and halide anion, at
least in a polar aprotic solvent, in equilibrium with undissociated halide, is far more satisfactory, with the equilibrium shifting as the
carbocations progressively bond electrophilically to the lone N electron pair on the tertiary amine. There is sufficient evidence of the formation, to
varying extents, of carbocations from alkyl and aryl halides in suitable solvents; this being only slight in primary alkyl halides, greater in
secondary and tertiary alkyl halides, and greatest in conjugated unsaturated halides and aryl halides in which the positive charge of the carbocation
can be delocalized by resonance (e.g. benzyl halides, and especially triphenylmethyl halides). Any reaction by the latter compounds with a tertiary
amine, involving a 5-coordinate reactive intermediate, would clearly be impossible for steric reasons.
Illustration: the SN2 "nucleophilic attack" mechanism propounded on the above web-page for formation of a quaternary ammonium salt.
[Edited on 5-4-10 by JohnWW]
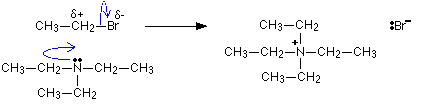
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JohnWW  | | The nucleophilic substitution mechanism propounded on this page, for successive replacement of the Hs in NH3 by alkyl groups from a polar alkyl halide
to form the primary, secondary, and tertiary amines, is clearly correct. This is because the substituted Hs can be easily removed as H+ by other NH3
molecules to form ammonium chloride or bromide. |
JohnWW, I don't think you fully understood the SN2 mechanism. These proton transfers on the reaction products are not part of the nucleophilic
substitution mechanism. The deprotonation is not synchronous with the substitution and thus has no influence on the SN2 activation energy. There are
plenty of good descriptions of SN2 substitution mechanism in most organic chemistry schoolbooks as it is one of the most important/common reactions in
organic synthesis. I suggest you take some time into understanding it fully as it is worth knowing at least some basics about it.
| Quote: | | HOWEVER, this cannot be said for the final formation of a quaternary ammonium salt from the tertiary amine by reaction with another alkyl halide
molecule, near the bottom of the page, simply because there is NOTHING on the tertiary amine to be nucleophilically "substituted" for, meaning that
this final step CANNOT be a genuine "nucleophilic substitution". |
It is as genuine SN2 as it can be. The nucleophile (Et3N) displaces the leaving group (bromide) from ethyl bromide. There is absolutely nothing
different in the form this reaction occurs when compared to all the other ammonia>ethylammonium>diethylammonium>triethylammonium sequences.
In each case you start with an N-nucleophile displacing the bromide on ethyl bromide electrophile and end up with a quaternary ammonium salt.
| Quote: | This part of the page reads as follows:
"A quaternary ammonium salt is an ammonium salt (for example, NH4+ Br-) in which all the hydrogens have been replaced by an alkyl group - for
example, (CH3CH2)4N+ Br-.
The mechanism
The tertiary amine still has an active lone pair on the nitrogen and, once again, that can attack the d+ carbon in the bromoethane.
But this time there is nowhere else for the reaction to go. There is no longer a hydrogen atom on the nitrogen that an ammonia molecule could remove,
and so the reaction finally comes to an end.
The product is a salt called tetraethylammonium bromide, (CH3CH2)4N+ Br-. "
This does not mention nucleophilic substitution, but it still implies nucleophilic "attack" by the lone electron pair on the N atom.
|
That text explains it well, but you must have misunderstood it because it clearly describes a normal SN2 substitution just as depicted on the scheme.
| Quote: | | However, in my view, this is unsatisfactory, because it implies the formation of a reactive intermediate in which the halide C atom has a coordination
number of 5 (at least 3 of which are to bulky atoms other than Hs), which is inconsistent with the available number of sp³ hybrid orbitals.
|
There is nothing wrong with that. SN2 substitutions indeed involve a pentacoordinated carbon. The incoming nucleophile interacts with the antibonding
sp3, while the bonding sp3 is being broken.
| Quote: | | This is why I consider that an alternative mechanism, invoking the slight degree of formation from an alkyl (or aryl) halide of a carbocation and
halide anion, at least in a polar aprotic solvent, in equilibrium with undissociated halide, is far more satisfactory, with the equilibrium shifting
as the carbocations progressively bond electrophilically to the lone N electron pair on the tertiary amine. |
Mechanism proposals must apply the Occam's razor rule and this does not. Besides, it is against the experimental evidence. We know that triethylamine
and ethyl bromide give tetraethylammonium bromide in protic nucleophilic solvents such as methanol and aprotic polar solvents such as DMSO. Therefore
there can be no SN1 mechanism involved, because if there would be a carbocation involved it would react with MeOH or DMSO. Besides, what is the point
in claiming a different mechanism without any evidence (or reason) when there is already a proven one available?
| Quote: | | There is sufficient evidence of the formation, to varying extents, of carbocations from alkyl and aryl halides in suitable solvents; this being only
slight in primary alkyl halides, greater in secondary and tertiary alkyl halides, and greatest in conjugated unsaturated halides and aryl halides ...
|
Could you please cite on such study where they observed the solvolysis of simple primary alkyl halides by SN1 mechanism? I'm not aware of any unless
they are benzylic or otherwise stabilized by the neighbouring group. (PS: Aryl halides can not form a carbocation - you probably meant benzyl
halides.)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I stand by everything Nicodem says above; the pentaco-ordinate transition state is standard for the Sn2 mechanism, and Clayden's Advanced Organic
Chemistry does a good job of showing and explaining the mechanistic features. Additionally there is a 5-coordinate carbocation, CH5(+), called a
"Carbonium ion", which can be formed in superacid media.
Not to confuse things here, but I read earlier today that the reagent combination MeF/SbF5 is a very vicious methylating agent, capable of methylating
even hydrocarbons, and excess MeF present in the reaction mixture! With SbF5 being a superacid, its quite possible (even likely) that this forms naked
CH3(+)...
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Oh, but has anyone actually succeeded in ISOLATING (e.g. by "freezing" the reaction by means of a liquid-N2-cooled "finger" protruding from a reaction
vessel), and then PROVING (e.g. IR-spectroscopically, mass-spectroscopically, NMR, or single-crystal X-ray diffraction) the existence of, any such
reactive intermediate, in alkylation of an amine, in which carbon is bonded to 5 other atoms, even CH5+ in which all 5 are Hs? And, as I noted above,
what about electrophilic additions to tertiary amines in which the incoming group is simply too BULKY to produce penta-coordination around a C (or an
N) atom (as well as some degree of ionization of it being energetically favored)?
[Edited on 8-4-10 by JohnWW]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JohnWW  | | Oh, but has anyone actually succeeded in ISOLATING (e.g. by "freezing" the reaction by means of a liquid-N2-cooled "finger" protruding from a reaction
vessel), and then PROVING (e.g. IR-spectroscopically, mass-spectroscopically, NMR, or single-crystal X-ray diffraction) the existence of, any such
reactive intermediate, in alkylation of an amine, in which carbon is bonded to 5 other atoms, even CH5+ in which all 5 are Hs?
|
There are no intermediates in SN2 substitutions. The reaction is a direct bimolecular transformation of the starting materials to the products going
trough the SN2 transition state. The SN1 substitution is the one going trough intermediates.
The pentacoordinated carbonium ions are not exactly the same thing, but as far as I know they were thoroughly researched by Olah and his group. If I
remember correctly they even have NMR spectra of these species. In SN2 transition state the carbon does not have five bonds, but is only reorganizing
orbitals to compensate for the breaking of one bond and formation of the other. The incoming nucleophile interacting with the antibonding orbital
weakens the bond of the carbon with the nucleofuge (leaving group). The Wikipedia entry actually does a good job in describing the mechanism (http://en.wikipedia.org/wiki/SN2_reaction), so it is pointless for me to go into details that I do not even remember any more. Similar
pentacoordinated state is also common in silicon atoms. Otherwise SN2 substitutions occur at other sp3 hybridized atoms as well, not just carbon and
group 14 elements.
| Quote: | | And, as I noted above, what about electrophilic additions to tertiary amines in which the incoming group is simply too BULKY to produce
penta-coordination around a C (or an N) atom (as well as some degree of ionization of it being energetically favored)? |
In the alkylations, the sterics of the nucleophile are less influential than the sterics of the electrophile, but it nevertheless has an important
influence. Bulky nucleophiles, for example the t-butoxide or diisopropylamine, can either react very slowly, fail to react at all, or even change the
course of the reaction (elimination, solvolysis, etc.). For example, even though tertiary amines have a higher electron density at the nitrogen, they
are slightly less nucleophilic than the corresponding secondary amines exactly due to steric reasons. The combination of sterics vs. electronics is
why the nucleophilicity decreases, for example, in the order of Et2NH>EtNH2>Et3N>NH3 rather than Et3N>Et2NH>EtNH2>NH3 (as expected
by purely electronic efects). Sterics can also completely block the reactivity of a nucleophile. For example, diisopropylethylamine does not react
with any other electrophile but a proton or BH3. All the others are too big to react with it and thus SN2 substitutions are not possible with this
amine. On the other hand, the bulkiness of the nucleophuge is more or less irrelevant as it posses no obstacle to the incoming nucleophile (just
consider the bulkiness of the iodide, tosylate or the leaving group in the Mitsunobo reaction).
[Edited on 8/4/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
OK, but I will believe it if/when I see it! And if Olah and his associates actually succeeded in obtaining, by some means e.g. "freezing" an
alkylation reaction, a reaction intermediate containing 5-COORDINATED and PENTAVALENT carbon (or nitrogen), and proving its existence by NMR, did he
get awarded the Nobel Prize For Chemistry, for disproving the validity of the solutions of the Schrödinger Equation and hence the whole of chemical
quantum mechanics, through proving that C (or N) can bond with MORE THAN THE FOUR 2sp³ hybrid orbitals??
The only additional bonding possibility might be through the 3s orbital; but it is at MUCH too high an energy level, certainly in C and N, to be
utilizable. If it were accessible, one would be able to obtain anionic species of second-period elements, isoelectronic with the Na atom, obtained by
electron-acceptance such as Ne- (the most likely one, but even more reactive than Na), F(2-) or at least HF-, and O(3-) or at least H2O-, and so on.
Also if it were possible, then the compound NF5 would also exist as a quantum-mechanics-violating pentacoordinate covalent compound; but all attempts
to synthesize it, or even as an ionic species [NF4]+F-, have failed, including in spite of attempts to trap it at very low temperatures from a
pressurized reaction vessel containing NF3 and F2. (And in spite of the existence of the cation as a salt of the strongest acids, such as NF4BF4 and
NF4ClO4 and NF4SbF6). So, in short, genuine pentacoordination of C or N is simply not possible, and any evidence of it just has to be mistaken.
[Edited on 9-4-10 by JohnWW]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
http://en.wikipedia.org/wiki/Carbonium_ion - references at the bottom of the page, eat your heart out!
Nicodem: If I remember correctly, the situation with pentacoordinate silicon is different; the silicon reagent forms an Ate complex with the
nucleophile, and then looses a group to give the overall impression of an Sn2 reaction.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
JohnWW, I still think the carbonium cations are something completely different and have no real similarity with the SN2 transition state. For one
thing, the carboniums are chemical species and not transition states. They are not truly pentacoordinated either, only the exchangeability of
hydrogens makes them appear such, while the geometry is that of a carbocation coordinated to the sigma bond of H2 (therefore nothing to do with the
interaction with the antibonding sp3 bond). Thus we should not be comparing carbonium ions with the SN2 transition state.
Quote: Originally posted by DJF90  | | Nicodem: If I remember correctly, the situation with pentacoordinate silicon is different; the silicon reagent forms an Ate complex with the
nucleophile, and then looses a group to give the overall impression of an Sn2 reaction. |
I agree. I was actually thought about it being a ligand exchange based nucleophilic substitution on the ate intermediate, but here I got into doubts
because Si has no vacant orbitals like the metals forming metalates. This got me to the wrong conclusion. Some day I will have to read about silicon
chemistry as well - sticking only to carbon and its neighbours is too incestuous.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JohnWW  | [...] did he get awarded the Nobel Prize For Chemistry, for disproving the validity of the solutions of the Schrödinger Equation and hence the whole
of chemical quantum mechanics, through proving that C (or N) can bond with MORE THAN THE FOUR 2sp³ hybrid orbitals??
[...]
The only additional bonding possibility might be through the 3s orbital; but it is at MUCH too high an energy level, certainly in C and N, to be
utilizable. |
From a purely physical point of view, two things about such a state are clear to me, that it's
both excited and metastable. The transition from such a state to the final state is a decay process, just like any other emission process. In
particular, I'd have to assume initially that this process obeys Fermi's golden rule about unit transition probabilities. As a decay process, you're
moving from an excited state to a lower-energy state (possibly a ground state). The excited state is going to look like a bonded state on first
examination, with the difference that there are lower-energy states available and that it's unstable. But that doesn't mean there's not an eigenvector
for that configuration.
In the present reaction, I can make a reasoned guess about what the molecular orbital process looks like. For the N atom, it has its typical sp3
hybrid orbitals. This is nice-and-stable, and so on collision with the soon-to-be 5-coordinated C, a couple of electrons from the N move onto it.
They'll move into the one of the sp3 orbitals of the C and displace another electron pair to a higher energy state (by Pauli exclusion, essentially).
Since these are interchangeable particles, I won't get caught up defending anything but the net effect, which is to push one of the existing C bonds
into a higher energy state. To be concrete, say it's one of the C-H bonds, originally a sp3 orbital with n=2, to sp3 with n=3. (I must say I'm
unfamiliar with the non-ground state solutions here and their notation, but the particular excited state doesn't matter to the ultimate issue.) Now
you have a non-ground state bond that is going to decay. Note that such an excited bond has a higher expected value of the interatomic distance ("bond
length" for a stable bond), so while the C is 5-coordinated, it's not in an ordinary bipyramid, but some skewed thing, with a good likelihood that the
expected bond angle between the two hydrogen atoms is small. By assumption, the two electron pairs are in different states (one excited and one not),
so Pauli exclusion doesn't apply and there can be significant spatial overlap between the bonds, hence a smaller-than-usual bond angle. So one
possibility for the intermediate shape is a perturbed tetrahedron, with one vertex doubled up and split apart a bit. But back to the decay. Assuming
an excited C-H bond, when it decays, it does so to an sp3, pushing out the sp3 electrons in the bond to the Br. (Again, I'm only defending the net
effect.)
The core of this argument is that the 5-coordinated C has 4 2sp3 bonds, in their ground states, and 1 3sp3 bond, which is excited. There's no
violation of Pauli exclusion (which would be the case with 5 2sp3 bonds simultaneously). There's no angular momentum problem, because you have 2sp3
--> 3sp3 and 3sp3 --> 2p. The activation energy of the reaction will correspond to the net energy to excite the presumed C-H bond (less the
energy gained by the formation of the C-N one).
|
|
|
|