hstiglitz80
Harmless

Posts: 1
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
1,3 - dimethylbarbituric acid, any good uses?
happened across some 1,3 - dimethylbarbituric acid. any good uses/syntheses from this?
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
I am going out on a limb here, but by "good uses/synthesis" I am guessing you mean are there any neet drugs you can make from this... wrong place. And
if you are going to ask such a question you should at least say what you have in mind.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Well look at the structure and see what obvious reactions you can do. To the amide bonds in particular.
You can hydrolyze it into malonic acid and N,N'-dimethylurea which can probably be decomposed into methylamine.
You can then use the malonic acid for an oscillating reaction!
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
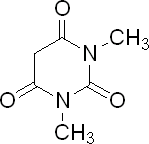
Barbiturates, including 1,3 - dimethylbarbituric acid (which is sold by Sigma-Aldrich, but is not available in Canada because it is a controled drug
there), also known as N,N'-Dimethylbarbituric acid, as central nervous system depressants, are mostly powerful sedatives and sleep-inducing hypnotics.
Although not giving a physiological "high", they can be addictive. They were first prepared by Bayer in 1863 in Germany, starting with malonic acid,
and by 1903 were being used as drugs.
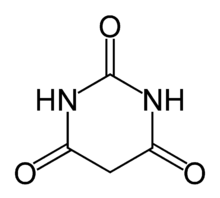
See http://en.wikipedia.org/wiki/Barbiturate . They are derivatives of "barbituric acid" (see image), which has no worthwhile pharmacological effects
of its own, but which, when dimethylated, has some structural affinity with caffeine. Adding two methyl groups, in place of the Hs on the Ns, would
eliminate the acidity of the compound.
[Edited on 24-3-10 by JohnWW]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Yes, interesting syntheses, definitely! It can easily be dialkylated at the 5-position giving barbiturate (the sleeping-aids) derivates. Either with a
sterically hindered organic base or with K2CO3/PTC. I'm sure you can find the references. If you're feeling adventurous, you could for example try
K2CO3/DMF. I have no idea if these N,N'-dimethyl barbiturates are biologically active. In case they are, the pharmacology is probably uninteresting
(IMHO), but interesting chemical transformation nonetheless.
| Quote: | | Barbiturates, including 1,3 - dimethylbarbituric acid [...] are powerful sedatives and sleep-inducing hypnotics. |
You sure? I thought the sleeping-aid barbiturates were the 5,5-dialkylated ones...?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Try a Google search on it.
I did and got dozens of references but nothing that was really interesting.
|
|
|