CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
4-Allylcatechol Methylenation
A 4-allylcatechol methylenation reaction was attempted using the results from my latest eugenol demethylation procedure using aryl (AlBr2)HBr complex
as demethylating reagent, which I believe is now yielding 4-allylcatechol at around 50% yield based on eugenol, give or take.
The methylenation method is similar to the method I used for protocatechualdehyde methylenation to piperonal I posted here in 2014, which itself was
based on the procedure referenced in "The Methylenation of Catechols"
W. Bonthrone and J. W. Cornforth
J. Chem. Soc. (C), 1202-1204 (1969)
There are four main differences with the approach I'll be using here.
1) I'll be using potassium carbonate as base instead of hydroxide (I've tried using sodium hydroxide twice previously, with almost no yield and
complete loss of 4-allylcatechol feedstock).
2) Instead of using an oil bath I'll be using a stirrer mantle and regulating the solution temperature by controlling the dichloromethane reflux, by
adding or removing dichloromethane as needed. The reaction temperature being carefully monitored to maintain 125 - 130 C at all times.
3) Vigorous overhead stirring will be used here since there is some phase separation.
4) The base & 4-allylcatechol will not be premixed beforehand, they will be added separately, a squirt at a time down the leibig condenser, using
two separate glass pipettes.
Also since 4-allylcatechol is oxygen sensitive and with the longer reaction time, argon atmosphere will be used.

Reagent ratios:
250 ml DMSO
55 ml dichloromethane
73.5 g K2CO3 (in 73.5 g dH2O)
68 g crude 4-allylcatechol crystal mass
Procedure
25 ml DCM (dichloromethane) is added to the DMSO in the 500 ml RBF, this is just enough to allow a good reflux at 125-130 C. The remaining 20 ml will
be added as needed to maintain reaction temperature within the target range. Approximately 1 ml DCM will reduce the reaction temperature by a degree
C, so it will be replaced as its slowly consumed by the reaction.
With solution temperature measurement and condenser fitted in place, heating and vigorous overhead stirring is turned on and allowed to reach reflux.
4-AC, DCM & K2CO3 addition solution.

The 4-AC is dark coloured due to some air exposure, all of my 4-AC samples/solutions rapidly turn pink, violet then blue (when anhydrous) when exposed
to air. In the presence of moisture it would instead turn brown and eventually black. The 4-AC will be melted by gentle heating prior to addition.
With the refluxing RBF solution at 125 C (refluxing at ~2 drips/second), and the mantle on constant 80% heating, the addition is started with a few
ml of the K2CO3, followed with about a ml of the 4-AC. Repeating a few times, then allowing to stir for 10 minutes, before continuing. Going slow, 3
hours was given for the addition, stirring until all DCM has been added, and with another 2 hours stirring after addition.
At about 90 minutes into the addition, a strong root beer odour was noted from the addition pipette(s).
Pipette addition was as far down the condenser as possible, to ensure the DCM reflux carries the reactants into the reaction mixture instead of
accumulating solids in the condenser.

Nearing the end of the addition now.

The black marker lines represent the level before addition was started, to help judge the addition proportions.
Last of the 4-AC crystals, will be melted by gentle external heating with a hot air gun before addition with the pipette.

5 hrs in, reaction mixture is dark brown.

The whole reaction mixture was combined with tap water and steam distilled.
Pressure cooker was used to supply the steam.

The first product to come over was a little leftover dichloromethane, and then lots of free oil on the sides and sinking to the bottom.


A total of 1.7 L of condensate was collected, and 33 g of free oil (inside amber bottle). No solvent extraction was carried out on any of the
condensate. Steam distillation was stopped at this point because there was a lot of waxy crystalline non-volatiles coming over and accumulating in the
condenser, so it was assumed the target product was mostly distilled.

The 33 g of oil was then vacuum distilled.

First fraction, collected up to 116 C. Vacuum distillation was stopped to test the oil to decide when to change receivers. Pure root beer oil.

Second fraction, collected up to 125 C. Only 3 ml collected, possibly iso.

Third fraction collected from 125 C to 147 C when solids began accumulating in the condenser. Distillation was stopped at this point. Temperature
increased rapidly after the second fraction, to 147 C, so am confident a clean fraction separation was achieved.
Fraction in receivers one and two were combined,
total isolated yield was 15.46 g of 5-allyl-1,3-benzodioxol. Seems quite pure but haven't confirmed purity yet.
The purity of the 4-AC was unknown so it remains impossible to calculate the exact molar yield.




[Edited on 14-2-2019 by CycloKnight]
|
|
|
monolithic
Hazard to Others
  
Posts: 435
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Another interesting write-up, thank you for your contributions.  Just curious,
have you taken a look at the paper I posted in your other thread, regarding eugneol demethylation with aluminum isopropoxide? Just curious,
have you taken a look at the paper I posted in your other thread, regarding eugneol demethylation with aluminum isopropoxide?
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Allowing for impurities in the 4-AC, I'd estimate molar yield to be somewhere in the 25-30% region.
The reason for adding the base and 4-AC separately, is because when mixed (even with DMSO added) there is some phase separation, and the 4-AC low
melting point (~49 C) makes it easy to melt and add in liquid in form without having to dissolve (as is needed with protocatechualdehyde).
The reason for avoiding the oil bath is because the oil is messy, and I've found from previous experiments that my DCM reflux method seems to work as
well, but this will be tested later with more highly refined 4-AC. I've had purer 4-AC, but I'm afraid a lot of it was destroyed. Its sacrifice was
not in vain however, as it has somewhat expanded my knowledge of what doesn't work as far as 4-AC methylenations go.
For the reactant concentrations used in this run, the reaction temperature increases by about a degree every 10 minutes, indicating about a ml of
dichloromethane being consumed. It perhaps comes as no surprise that this is considerably slower than the hydroxide equivalent in DMSO (with
protocatechualdehyde). Its important to maintain as high a dilution as is practicable, to maximize yields and minimize side products. So next time a
suggestion might be to pace the 4-AC addition with the dichloromethane consumption. This would lengthen the reaction time somewhat, however.
Monolithic, I have - it looks interesting! There are a few demethylation routes that look to hold promise but tbh I've been that busy formulating this
bromine method (optimizing rxn conditions, bromide recovery, etc) that I haven't yet tested any other routes since working on this one. This 4-AC
method requires two days (with reagents in hand), one day to form the demethylating reagent, run the eugenol demethylation and hot hydrolysis. The
second day to do the workup, involving solvent removal under vacuum (about 2 hrs) and the vacuum distillation collecting the 4-AC fraction.
I haven't quite finished the final writeup on the method yet but when I do I'll post the update on the eugenol demethylation thread.
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
As an aside, this thread was intended in the interest and spirit of chemistry discussion only. Not interested in discussing any theoretical misuse of
this (albeit synthetically replicated) wonderful naturally occurring substance and traditional root beer flavouring. I have it growing in my
greenhouse, piper auritum. I posted a thread about it a while back.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2693
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Really nice work! I wonder why NaOH was such a problem. Possibly the anion is not so stable -- if the benzylic carbon is deprotonated, I could imagine
an isomerization all the way to this:
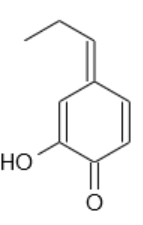
(Edit by Texium: removed legality discourse)
[Edited on 10-30-2019 by Texium (zts16)]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Quote: Originally posted by clearly_not_atara  | I wonder why NaOH was such a problem. Possibly the anion is not so stable -- if the benzylic carbon is deprotonated, I could imagine an isomerization
all the way to this: <snip>
|
I'm curious also, if there was any tar generated then I certainly didn't notice it. I did observe of a lot of waxy crystalline product which appeared
similar to what is also produced using the K2CO3 method (but as a side product) from early on in the steam distillation, indicating there was little
methylenated product. I'd estimate there were just a few drops of methylenated product, from 58 g of 4-AC. Any more experiments with NaOH will be on
the mmol scale.
It seems that the 4-AC didn't polymerise or get degraded, but only yielded a different main product, which would tie in with what you're suggesting.
Interestingly, the run with the 4-AC & NaOH was possibly going to be the SM post, so took lots of images.
Distilling the final fraction during the workup. The secondary still head saves a lot of agro by avoiding the condenser altogether (receiver just gets
swivelled downwards when ready to collect the last fraction).


Fully crystallised.

Melted in preparation for the methylenation (purple caused by oxygen exposure)

Just a shame it didn't work with NaOH. When I get time to prepare more, I'll aim to use similar quality 4-AC and repeat the methylenation experiment.
[Edited on 16-2-2019 by CycloKnight]
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Update, just to post a correction. In my last post I mentioned that methylenation failed when using NaOH. I've learned that it does indeed work and is
quicker and generally easier than using K2CO3. The last 4-AC I prepared using a new variation (dehydrobromination via ester formation and hydrolysis)
yielded nearly 3 ml of our favorite root beer flavoring from 7 g 4-AC, NaOH was used as base. Reaction was completed in about 1 hour.
I believe what may have happened, was that when I'd tried using NaOH without success, the previous dehydrobromination step had failed without my
realizing, and dehydrobromination occurring during the methylenation step with NaOH, yielding the alcohol (1-(1,3-Benzodioxol-5-yl)-2-propanol, mp ~70
deg C) instead of the allyl oil. This would explain the crystalline white solids in the steam distillate, but only I'd assumed they were a dimmer side
product.
|
|
|
Texium
|
Thread Split
30-10-2019 at 08:07 |
Mar-Vell
Harmless

Posts: 8
Registered: 31-8-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CycloKnight  | | The last 4-AC I prepared using a new variation (dehydrobromination via ester formation and hydrolysis) yielded nearly 3 ml of our favorite root beer
flavoring from 7 g 4-AC |
Are you saying here that you reacted eugenol bromide with say sodium acetate (in a dipolar aprotic solvent?), formed eugenol acetate, and then
hydrolyzed that to eugenol hydroxide? If so, how did you go from eugenol hydroxide to 4-AC? (A detailed experimental write-up would be very generous
of you.)
Quote: Originally posted by CycloKnight  | | I believe what may have happened, was that when I'd tried using NaOH without success, the previous dehydrobromination step had failed without my
realizing, and dehydrobromination occurring during the methylenation step with NaOH, yielding the alcohol (1-(1,3-Benzodioxol-5-yl)-2-propanol, mp ~70
deg C) instead of the allyl oil. This would explain the crystalline white solids in the steam distillate, but only I'd assumed they were a dimmer side
product. |
This is a good educated guess, but of course, without experimental data, you can't really know what actually happened. You may want to just straight
up synthesize some of the 1-(1,3-Benzodioxol-5-yl)-2-propanol to determine what its properties are. I certainly would very much like to know the
results of such an experiment and encourage you to pursue this line of inquiry.
Please keep up the great work.
[Edited on 28-11-2019 by Mar-Vell]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by CycloKnight  | | As an aside, this thread was intended in the interest and spirit of chemistry discussion only. Not interested in discussing any theoretical misuse of
this (albeit synthetically replicated) wonderful naturally occurring substance and traditional root beer flavouring. I have it growing in my
greenhouse, piper auritum. I posted a thread about it a while back. |
How rich are the leaves of the hoja Santa in root beer oil?
I don't like licorice but the idea of a candy shop flavour appeals to me.
Are they a nice flavour? Is it as described? Is growing the plant worth it to get some flavouring?
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by monolithic  | Another interesting write-up, thank you for your contributions.  Just curious,
have you taken a look at the paper I posted in your other thread, regarding eugneol demethylation with aluminum isopropoxide? Just curious,
have you taken a look at the paper I posted in your other thread, regarding eugneol demethylation with aluminum isopropoxide? |
Aluminium isopropoxide sounds a lot easier than bromine,inert atmosphere and Lewis acids.
|
|
|
Mar-Vell
Harmless

Posts: 8
Registered: 31-8-2012
Member Is Offline
Mood: No Mood
|
|
What about demethylating eugenol acetate or eugenol hydroxide?
CylcoKnight, have you tried to demethylate either eugenol acetate or eugenol hydroxide?
I think that a demethylated product with desirable stability characteristics like catechol and protocatechualdehyde might be useful. Something which
is not (very) sensitive to basic conditions and which is stable at room temperature and regular air conditions. That way it can sit around for awhile
without decomposing, be recrystallized and cleaned up without the need for vac distillation, or be cleaned up by a dilute sodium hydroxide extraction
and solvent wash (like with eugenol).
The demethylated products of eugenol hydroxide or or eugenol acetate can then be methyleneated at the catechol region, and then the functional group
on the side chain could be converted into a terminal alkene, perhaps through hydrolysis and dehydration reactions, so that you can ultimately have
your root beer flavouring, if you so desire.
I personally am interested in these compounds because their catechol nature is useful for polymerization reactions related to adhesives. So, really,
what's on the propane side chain may not be so consequential.
[Edited on 28-11-2019 by Mar-Vell]
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Quote: Originally posted by Mar-Vell  |
Are you saying here that you reacted eugenol bromide with say sodium acetate (in a dipolar aprotic solvent?), formed eugenol acetate, and then
hydrolyzed that to eugenol hydroxide? If so, how did you go from eugenol hydroxide to 4-AC?
|
Yes, except carried out after demethylation. This was roughly covered in the demethylation thread (methanol was used as solvent with sodium acetate),
after KOH alcohol ester hydrolysis the dehydration step is achieved by heating with KHSO4. During the previous run, finely ground KHSO4 was merely
added prior to the vacuum distillation, and dehydration / vac distillation was completed in one step. Water vapor comes off, then the alkene/allyl
product distills. Temperature is needed for this step, so a lesser vacuum was used.
However, rather than proceeding down this route to the alcohol via ester, next time I will try and go back to KOH (aqueous / methanol), since the end
product is the same, and the ester route does cause additional losses. I was originally trying to find an efficient single dehydrobromination step for
bromoeugenol to the alkene (anhydrous methanol / KOH wasn't working, even after a several hour reflux), and then just started experimenting with the
ester route after it was suggested. If aqueous alcoholic KOH can do it also (only tried anhydrous reflux so far), then that'll be easier and no doubt
quicker, now that KHSO4 dehydration seems to work for reforming the alkene/allyl group.
Later on I plan to try converting the bromoeugenol to the alcohol (as described above), and then proceed to the demethylation. Therefore less time
spent processing the fickle diol, and subsequent losses. Previous runs have been spent trying to dial in the demethylation reaction parameters, rather
than reduce decomposition. Though I have a few alternative routes (also using isoeugenol I prepared) on the to-do list after I complete another chem
project still underway.
[Edited on 30-11-2019 by CycloKnight]
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Quote: Originally posted by draculic acid69  |
How rich are the leaves of the hoja Santa in root beer oil?
I don't like licorice but the idea of a candy shop flavour appeals to me.
Are they a nice flavour? Is it as described? Is growing the plant worth it to get some flavouring? |
I detailed a piper auritum oil steam distillation in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=90636#...
I managed about 1.5% oil based on dried leaf mass, however I do have another mini project involving the sacred herb. I have about a dozen plants in
soil pots at the moment, and I aim to propagate them to about 50 pots by the summer. That way, they can be grown and then harvested all at once, to
yield enough fresh leaf for a worthwhile steam distillation, no leaf drying required (due to losses). Give it a few weeks, and then harvest and steam
distill again, etc etc.
The oil is quite amazing, and easy to steam distill with the leaf in hand.
The only downside is that a lot of leaf is needed, but it is sustainable at least.
|
|
|
|