ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
Carbamate Synthesis
Hi everyone,
I am interested in synthesizing a carbamate, 3-methyl-3-pentanol carbamate, to be exact. Since this is an ester, would I be able to react Carbamic
Acid with 3-methyl-3-pentanol, using sulphuric acid as a catalyst, to create my 3-methyl-3-pentanol carbamate product? ...Just how you can react
ethanol and acetic acid, using sulphuric acid as a catalyst, to create ethyl acetate...do the same rules apply?
...I hope so!
Anyone know? Thanks for the help!
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
You have carbamic acid?
As I understand it carbamic acid is quite unstable, and certainly wouldn't survive any refluxing. Making carbamates usually requires very nasty
chemicals which are often times exceedingly toxic. However if you have access to the proper equipment and chemicals there are a few different routes
you can take. Alcohols react with alkyl isocyanates in the presence of a catalyst to from N-substituted carbamates. There is however another procedure
which might work the Curtius rearrangement, which involves alkanoyl azides which upon heating (Carefull!) rearrange to isocyantes. Take a look: http://en.wikipedia.org/wiki/Curtius_Rearrangement.
I hope that helps!
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
For more information on this substance, see its generic name Emylcamate or its trade name Striatran.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
You can prepare it by reacting phosgene with the alcohol and then ammonolysis of the alkyl chloroformate, this is how it would have been done
industrially.
Two alternative approaches are here;
http://pubs.acs.org/doi/abs/10.1021/jo01047a033
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Free carbamic acids are unstable and fall apart, yeilding the free amine and carbon dioxide. Carbamates can be made from the isocyanate and an
alcohol, or a chloroformate and an amine, amongst other methods.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
There was some small business that manufactured a catalyst that allowed N-methyl carbamate esters to be synthesized from alcohols and urea.
Unfortunately, it's a proprietary catalyst and the guy was very guarded about giving information about it!
But generally, if you want to work with carbamates, you're starting off with rather dangerous reagents (phosgene, methyl isocyanate, etc.) and making
a potentially more dangerous, neurotoxic product. The only redeeming value about carbamate poisoning over organophosphate poisoning is that
the morbidity isn't as great if you do survive.
EDIT: well, it seems this is a very different kind of carbamate than the kind I was thinking of! Carry on.
[Edited on 11-30-10 by DDTea]
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
If I was doing it on a small scale I would go for the reaction of the alcohol with phenyl chloroformate to prepare (3-methyl-3-pentyl) phenyl
carbonate.
I would then react that with ammonia to make the carbamate. The phenyl group is a lot better as a leaving group so you would get the product in high
purity.
This is a literature method for preparing this particular type of carbamate on a research scale so it will be documented with reaction conditions,
etc.
As far as I know it is obsolete as a pharmaceutical but I assume that you are making it on a research basis and have access to the reagents and
equipment required.
|
|
|
ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
Thanks, I do have information on this compound already. I have the Merck Index as a program on my computer. Here is what it says about Emylcamate:
Monograph Number: 3598
Title: Emylcamate
CAS Registry Number: 78-28-4
CAS Name: 3-Methyl-3-pentanol carbamate
Additional Names: 1-ethyl-1-methylpropyl carbamate; diethyl methyl carbinol urethan; 3-methyl-3-pentyl carbamate; methyl diethyl carbinol urethan;
tert-hexanol carbamate
Manufacturers' Codes: Kabi 925; JD-91
Trademarks: Nuncital (Kabi); Restetal (Kabi); Statran (Delagrange); Striatran (Merck & Co.)
Molecular Formula: C7H15NO2
Molecular Weight: 145.20.
Percent Composition: C 57.90%, H 10.41%, N 9.65%, O 22.04%
Literature References: Prepn: DE 245491 (1912 to Chinifabr. Zimmer); DE 254472 (1912 to E. Merck); Melander, Hanshoff, US 2972564 (1961 to Kabi).
Properties: Needles from 30% ethanol, mp 56-58.5°. bp1.0 35°; bp0.7 24°. Slight odor of camphor. Soly in water: 4.0 mg/ml. Freely sol in
alcohol, ether, benzene, glycol ethers.
Melting point: mp 56-58.5°
Boiling point: bp1.0 35°; bp0.7 24°
Therap-Cat: Anxiolytic.
_____________________________________________
Anyway, thanks for all the replies! I didnt realize before that synthesizing this compound would be that difficult, and use such dangerous chemicals.
Those azides scare me, as well as highly toxic gases.
I want to synthesize this compound for research, correct. I have access to chemicals, but I don’t have any on hand. I can acquire lab chemicals
quite easily though, except the more exotic and restricted ones. So far ScienceSquirrel has listed a method that I favour, that doesn’t involve
anything too dangerous. Making this compound using this method in a small scale is my goal. I can’t find any literature on the method though. Is
there a detailed general procedure for emylcarmate's synthesis or for a similar carbamate that you can share with me? If no please point me in the
right direction so I can search and find it. Thanks!
So basically in the method (or correct me if I’m wrong) the 3-methyl-3-pentanol will react with the phenyl chloroformate to make the products of HCl
and (3-methyl-3-pentyl) phenyl carbonate. Then the (3-methyl-3-pentyl) phenyl carbonate reacts with ammonia to form the emylcarmate and phenol?
(I am still learning organic chemistry, as you can tell. I love this field of study.)
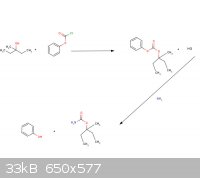
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
This paper has a method and there are references in it to the chloroformate method.
http://pubs.acs.org/doi/abs/10.1021/jo01047a033
|
|
|
Xylitol
Harmless

Posts: 15
Registered: 8-10-2015
Member Is Offline
Mood: No Mood
|
|
I am also interested in this synthesis, but I am a beginner and I'd like some clarification before proceeding.
I can't find much info on the chloroformate method, and the link to that research article only once mentions this chemical on the first page, but
doesn't actually talk about synthesis.
Would a beginner doing this reaction the more common way (sodium cyanate and triflouroacetic acid) be difficult? I understand these chemicals are very
toxic, but is the purification of the final product trivial or do you think it may be hard to remove the toxic products?
The process I would follow is detailed in this patent: https://www.google.com/patents/US3072710
| Quote: |
Example 1 A mixture of 4.0 g. of 3-methyl-3-pentanol, 5.2 g. of sodium cyanate, 9.1 g. of trifiuoroacetic acid and 50 m1. of tetrahydrofuran is
stirred at 45 C. for two hours then neutralized by the addition of solid sodium carbonate. The mixture is concentrated, treated with water and
filtered to give 3-methyl-3-pentyl carbamate which has tranquilizing activity. |
Also are there any steps you think might be overlooked? Because I'm a beginner I need the most detailed explanation. I'm thinking one thing that might
be is the 3-methyl-3-pentanol and sodium cyanate should probably be mixed first with the trifluoroacetic acid added dropwise?
|
|
|
softbeard
Hazard to Self
 
Posts: 69
Registered: 23-7-2013
Member Is Offline
Mood: moody
|
|
Fortunately for you, neither sodium cyanate nor trifluoroacetic acid are toxic. Trifluoroacetic acid is a powerful acid and a corrosive/irritant, but
that's different. Put another way, the trifluoroacetate anion has a low order of toxicity. Similarly, cyanate has a low order of toxicity. Don't
confuse cyanate with cyanide; it's very different.
I can't help you much with your reaction, but if sodium cyanate and trifluoroacetic are all you're adding to your 3-methyl-3-pentanol, I don't think
you need worry about poisonous chemicals. Just take standard precautions against irritants, have good ventilation, and wear eye protection.
Let us know how it works out.
|
|
|
Xylitol
Harmless

Posts: 15
Registered: 8-10-2015
Member Is Offline
Mood: No Mood
|
|
I performed the reaction and boiled off/evaporated as much solvent as possible. The resulting liquid crystallized. I tried adding hot water and
cooling to recrystallize, but nothing seemed to happen, so I just boiled off the water and I'm left with the crystals again. Should I try again with
another solvent?
Anyway, I'd really like to know what the by products are in this reaction. Clearly there should be some sodium trifluoroacetate after I neutralized it
with sodium bicarbonate. What other by products will form from the sodium cyanate and trifluoroacetic acid?
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I was on a wiki walk earlier and came across the ethyl carbamate page. https://en.wikipedia.org/wiki/Ethyl_carbamate
It claims that ethyl carbamate can be synthesized from ethanol and urea and that the reaction is "much faster" at higher temperatures. It does not,
however, specify how much faster or how high the temperature has to be, and is unreferenced. Personally, I think it sounds like a load of bunk, but
I'm not familiar with the properties of carbamates.
DDTea mentions upthread that a catalyst is required for the reaction, so I suppose that Wikipedia's claim is too good to be true, but I wanted to make
sure anyway.
|
|
|