| Pages:
1
2 |
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Safe preparation of lead oxides
So I've been toying with the idea of making red pigment from lead - either lead tetroxide, aka "red lead" aka minium, or lead(II) oxide in its
"letharge" form. They can apparently be formed by burning lead metal in air at certain temperatures.
I can easily get my hands on lead metal, and I can also fairly easily, uh, catch things on fire, but given the toxicity of lead I'm assuming just
burning lead metal out in the open is probably not a great idea. And even if I did prepare them, I would need to grind the crystals formed up in a
mortar and pestle, a process that would release lead oxide dust into the air that you'd definitely not want to breathe in and that my dust masks are
probably not sufficient to block.
Can lead oxides still be prepared relatively safely, given certain precautions, or should I just not bother?
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
You can make them at lower temperatures by melting lead with K/Na nitrate (not sure about other nitrates, guess the corresponding nitrite should not
be explosive.) Doing this in well ventilated area with some PP (oxide dust flies around) should be safe.
I have done this but I have never got the nice pure red oxide, it is just ugly orange, and purifying gets quite messy. That is why I did not try a
lot.
[Edited on 26-8-2019 by TheMrbunGee]

|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
So the one on the left is your own attempt and the one on the right was purchased, or what?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
It looks like PbO on the left and my Pb3O4 is uglier...it's not easy, the dry way...IIRC all my PbO came from heating the precipitated carbonate...the
CO2 may have kicked up dust...I can't remember for some reason.
There is (Blanchard says) a mostly wet and probably better way to make Pb3O4, from PbO and PbO2 in aq. NaOH.
[Edited on 27-8-2019 by S.C. Wack]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
On the right is purchased, yes and on the left is my attempt of melting lead metal and NaNO3, but I was after NaNO2 so I just collected all of the
lead oxides as they were, so - impure. Not the process If purity and color is important.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i would suggest using some kind of a high temperature filter like melamine sponge when decomposing toxic compounds
ive had my fun with nickel poisoning just from a steel pot where i evaporated a liquid containing insoluble nickel oxalate, at the very least consider
supplements that inhibits uptake of the specific heavymetal, for mercury that would be selenium
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Antiswat
||
|| i would suggest using some kind of a high temperature filter like melamine sponge when decomposing toxic compounds
|| ive had my fun with nickel poisoning just from a steel pot where i evaporated a liquid containing insoluble nickel oxalate, at the very least
consider supplements that inhibits uptake of the specific heavymetal, for mercury that would be selenium
maybe just do it in a fumehood or in the open, not in the kitchen
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Okay, so I've ordered some graphite crucibles and I have a propane blowtorch (for a number of projects - not specifically lead). IF I decide to go
through with trying to make lead oxides, based on the answers in this thread, am I correct in concluding that no safety precautions are necessary
besides wearing a dusk mask/respirator and optional lead uptake inhibitor supplements?
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Potentially a graphite crucible will react with lead oxide, nitrates, nitrites and most oxidizing salts. That may be a minor problem to the
clay/graphite crucibles if they are mostly clay.
I suggest you use a seamless steel can or a stainless steel dish.
From memory the type of lead oxide produced when heating lead is dependent on the temperature. I suggest you find a procedure and follow that.
From wiki: Lead(II,IV) oxide is prepared by calcination of lead(II) oxide (PbO; also called litharge) in air at about 450-480 °C:[4]
6 PbO + O2 → 2 Pb3O4
The resulting material is contaminated with PbO. If a pure compound is desired, PbO can be removed by a potassium hydroxide solution:
There is an elaborate prodedure for it in prepchem:
http://www.prepchem.com/synthesis-of-lead-tetroxide/
[Edited on 9/7/2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
If we are in the Responsible practices thread anyway then it may not seem too odd when I say: why not draw the line and simply stop playing with Pb,
Cd and Hg?
I mean collecting the elements is OK (in reasonably small ammounts), maybe some electrochemistry on Pb and Hg anodes may slip through as well, but
deliberately making more or less soluble compounds of these metals is irresponsible I think.
We are pursuing a hobby and our lives or survival of our families are not dependent on it. So we can say that no, I don't do this or that.  E.g. I don't mess with amalgam reductions. If a synthesis depends on it then I don't
do that synth or try to find an alternative. If a recipe calls for Pb-nitrate or -acetate to coagulate proteins then I try to achieve the same effect
by using zinc- or copper-salts. (Although copper is just slightly better than the mentioned three.) I refused to take a kilo CdSO4, though it was
literally for pennies. But why would I stockpile such a compound? E.g. I don't mess with amalgam reductions. If a synthesis depends on it then I don't
do that synth or try to find an alternative. If a recipe calls for Pb-nitrate or -acetate to coagulate proteins then I try to achieve the same effect
by using zinc- or copper-salts. (Although copper is just slightly better than the mentioned three.) I refused to take a kilo CdSO4, though it was
literally for pennies. But why would I stockpile such a compound?
Why would I take the risk of accidental slow poisoning myself via inhalation of dusts, vapours, or via skin contact of these compounds? These pretty
much "one way" compounds: they enter the body, then they don't want to leave.
For each of his own, but in the Responsible practices thread this opinion may find some open ears.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
When you consider that its not so long again every time you used 50l of gasoline in your car you had spread up to 7.5g lead a round or the red lead
and white lead at one time used in paint and the leaded solder in old electronics. Lead is still used in lead acid batteries , roofing, bullets,
pellets, fishing weights and even hair dye. Then it is probably no more irresponsible than all that and a lot less irresponsible than the previous
use of Arsenic and hexavalent chromium to preserve wood used in children's play parks.
I am not advocating irresponsible use of lead. Any usage will inevitably increase human exposure above background levels but I am suggesting its
important to have a sense of proportion about it.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
While working on lead dioxide electrodeposition topics I noticed that reacting sodium plumbite solutions with a moderate concentration H2O2 tends to
to give the "red lead oxide"percipitate. Plumbite can be made by dissolving lead(II)oxide PbO in alkali solutions. Lead metal also tends to form
plumbite in strong alkali solutions, but the reaction is veeeeery slow.
If one needs only a small amount then perhaps this method might be useful as it can be conducted without heating (if one starts with PbO) and the
release of lead fumes that accompany that process.
Either way, working with coupious amounts of lead compounds is not a healthy undertaking by any means.
Exact science is a figment of imagination.......
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Well, I got my graphite crucibles, so I chucked a couple lead drywall anchors in one and heated the bajeezus out of it (to what temperature I don't
know - it was hot enough to be completely liquid but not hot enough to glow). At first it was a pale yellow-gold color, and I swished the crucible
around a couple times to mix it moltem metal up and allow the lead not at the surface to be exposed to oxygen so it could form oxides. And at some
point, after some swooshing and at a very high temperature, it turned not yellow or red or orange, but... blue?
 
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by TheMrbunGee
||
|| You can make them at lower temperatures by melting lead with K/Na nitrate (not sure about other nitrates, guess the corresponding nitrite should
not be explosive.) Doing this in well ventilated area with some PP (oxide dust flies around) should be safe.
||
|| I have done this but I have never got the nice pure red oxide, it is just ugly orange, and purifying gets quite messy. That is why I did not try a
lot. | [Edited on 26-8-2019 by TheMrbunGee]
The blue color was probably caused by a thin oxide layer similar to the blue color on heated steel.
From wiki: "Steel turns blue because of a thin oxide layer that forms on the surface of the metal. The thin film interferes with light waves, which
enhances some wavelengths while reducing others"
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Welp, I tried making lead tetroxide by putting some lead carbonate I made earlier (from lead acetate (from lead metal + vinegar + hydrogen peroxide) +
calcium carbonate) in a crucible and heating to try to anneal it to lead tetroxide, since according to Wikipedia that's a thing. Just got an ugly pale
orangish (#e4bb87 is a good approximation) - certainly not scarlet, as the picture on Wikipedia would suggest.
So... another path is apparently lead acetate + potassium plumbate, for which I assume sodium plumbate is an acceptable substitute. And sodium
plumbate can apparently be made from lead dioxide in an NaOH solution, and lead dioxide can apparently be made by oxidizing lead acetate with NaClO.
So... dissolve lead in vinegar, add bleach, add NaOH, then add more lead acetate? Can anyone confirm this works?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
oxides color can vary a lot, just because it is an ugly orange it doesn't necessarily mean low quality
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Did somebody try to prepare Pb3O4 by the wet method as described in Brauer - K2Pb(OH)4 + K2Pb(OH)6 ? If you did, could you please share experience
also with the plumbites/plumbates preparation.
@Pumukli I think it is good to proof the information by citing some sources. Your phrase "they enter the body, then they don't want to leave" needs
some clarification. I found, that "adults typically absorb up to 20% of ingested inorganic lead after a meal and up to 60-80% on an empty stomach " .
95% of absorbed lead is stored in bones. "In times of stress (particularly pregnancy and lactation), the body can mobilize lead stores, thereby
increasing the level of lead in the blood" . "The body accumulates lead over a lifetime and normally releases it very slowly" - https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=9 . Thank you for make us aware about the lead toxicity but let me ask: could it be done in
some scientific way to make us able to get usefulness from it?
You didn't mention a special medicine which as far as I know is used in cases of lead poisoning to speed up the discharge of lead reserves in the body
- and it is a very interesting topic.
Also, how you propose to sort these metals - Hg, Cd, Pb in the order of increasing danger? So, if I plan to do experiments with them during my short
life which of them should I hold to the last days? (But I personally would prefer to discuss life and health issues in separate threads, it is
annoying to read it in threads dedicated to specific topics also because our friends who die from some diseases people mention here - like cancer or
poisoning - in 99.999% got them not from chemistry hobby).
[Edited on 22-10-2019 by teodor]
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Quote: Originally posted by Ubya  | | oxides color can vary a lot, just because it is an ugly orange it doesn't necessarily mean low quality |
It does if you intend to use it as a pigment, i.e specifically for its color.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Vorobyova suggests repeatedly boiling the product made from PbCO3 with a soluble Pb salt such as the acetate, before washing and drying.
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Okay, so I've have lead(II) acetate (concentration unknown; made from letting lead metal decompose in a jug of vinegar and hydrogen peroxide) and I
added bleach (5% - 10% NaClO according to the MSDS) expecting it to turn black or something given the typical color of PbO2. Instead it turned a
darkish orange once I added enough; it's not really the scarlet color I was expecting of Pb3O4, but apparently NaClO can decompose into NaOH, so if
the NaClO reduced the PbAc2 to PbO2, then some excess NaClO decomposed to NaOH which reacted with the PbO2 to yield Na2PbO4, which then reacted with
some other PbAc2 in solution... I guess Pb3O4 is a possible side product? Or is the orangeish color from the hexahydroxyplumbate ion or what? Keep in
mind this is all BEFORE adding NaOH.
I don't have a good handle on the reaction conditiona required to make PbO2 from PbAc2 (which I really want to get right, since PbO2 is an
intermediate for both lead tetraoxide and ammonium hexachloroplumbate, which I want to make). At such low concentrations, how much bleach would be
required? At what temperature? How long should I wait before adding NaOH to turn it into sodium plumbate, or do I add both the bleach and NaOH at the
same time?
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Did you try to follow Brauer procedure for PbO2 from PbAc2 , KOH and Ca(OCl)2, Arcaeca?
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
"I think it is good to proof the information by citing some sources."
What if I did not want to proof anything? I merely gave a hint of the possibility of chronic lead poisoning. Then anyone who want to play with these
compounds can look up the relevant facts. I did not bothered because I'm not interested in them.
I didn't mention any special medicine, yes. Because it is the "responsible practices" thread and if someone had been so reckless as to got poisoned by
these compounds - while played "responsibly" with them, then the proper treatment had been the responsibility of his/her doctors!
I don't propose to sort them either. For me they are practically in the same league: the stay-away league. If you asked me Hg is probably the worst
due to its highest vapour pressure at room temp and its ability to form toxic substances with various organic compounds. As I'm mostly into org. chem.
it is something to be worth taking into account.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I totally agree with you about the importance of making people aware of the danger, the difference is only I think to make them listening we should
provide some accurate information.
For example : how is it safe to mix lead salts with organic solvents. Based only on my poor chemical background I can imagine that it is not possible
to create lead-organic compound just by mixing, we should prepare at least grignard reagent to do the job. Experience of people who understand the
topic could help.
Also: volatility of salts, especially nitrates. It is in the range 100-150 C according to some sources. How is safe to do some reaction, for example
decomposing carbonate.
To make people aware we can only supply the information. We cannot say: do not do that. We can say: if you do that - you will get that etc.
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Sort of? I don't actually own the book but the procedure in the pdf hosted by this site uses CaOCl and KOH, neither of which I thought I could get my
hands on easily, so I was substituting in NaOCl (liquid bleach) and NaOH. However looking through an older thread (tid: 1932) apparently using bleach
is discouraged since it has NaOH and NaCl impurities that form side products. And it's come to my attention that CaOCl is sold as a pool chlorinator;
this is what they have at my local hardware store, but it's certainly far from pure; based on the MSDS could I expect a significant amount of side
product, or would it be better to just stick with the bleach?
 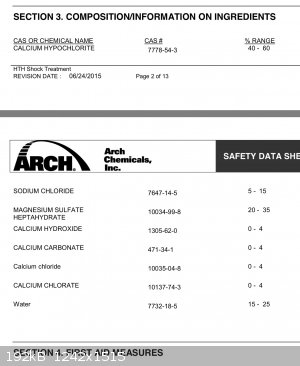
EDIT: Wait, apparently they did get it to work with bleach, but at 75 C for an hour, so I'll try doing it with a hot water bath instead of ambient
temperature later today.
[Edited on 10-23-2019 by Arcaeca]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
The book is in the SM library. http://library.sciencemadness.org/library/books/brauer_ocr.p...
Page 757.
For most reactions KOH/NaOH is not relevant, and I was wrong - according to the procedure - NaOH. Also, most of receipts that mention bleach or
Ca(OCl)2 work with a technical grade staff - it is so common that purification usually mentioned separately together with the procedure how to purify
it. Also, according to Brauer "technical grade Ca(OCl)2 (70-80% Cl) or from double this amount of technical grade bleacing powder". It says "boiled
for a few minutes".
|
|
|
| Pages:
1
2 |