Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
The unfindable reference for Acetone Dicarboxylic Acid Anhydride
Good day,
I am on the hunt for a reference for the synthesis of 1,3 Acetone dicarboxylic acid anhydride from 1940. Findlay makes reference to it as well as
Casale and a number of others. According to 1950's Findlay it yields "in excellent amounts"; that's the scientist equivalent of "BAD ASS DUDE!!!!111"
Here is the reference:
R. Kaushall, J. of Indian Chem. Soc., 17, 138 (1940).
Now, before anyone says UTFSE....
Google, sci-hub, my library at Uni.... cant find it. It definitely exists, but perhaps was never digitized? I've even emailed the current Indian Chem
Society about it (no response for 2 weeks now, not wholly unexpected....)
Can anyone here locate this beast? I'd even take a copy in Hindi at this point....
Thanks to all who try!~
[Edited on 3-2-2020 by Johnny Windchimes]
[Edited on 3-2-2020 by Johnny Windchimes]
|
|
|
Mush
National Hazard
   
Posts: 632
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
We have a section for article requests. Can you please contact Polverone or Woelen for access.
"References
Articles or books may be requested or shared in this forum. For access, please contact Polverone or woelen. "
It is def. offline.
Your Uni. should be able to get it for you for a small fee (about 5 USD) as an interlibary loan/request. If you are a student you should even have
discount on loans.
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Thanks I'll give that a go!
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Below is a more recent paper. Four steps 77% yield. What do you want it for?
Attachment: Acetone-Dicarboxylic-Acid-Anhydride.pdf (184kB)
This file has been downloaded 610 times
[Edited on 2/4/2020 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Hey there,
I'd very much like to stabilize it (it decomposes quickly as you may know) for storage. I've been having some issues with the various methods (From
Kato 1966 & 1967 to Findlay 1950 and Casale 1987) and I think its basically my poor temperature control. That, and the ungodly "classic" synth via
H2SO4 and Citric acid that I could barely handle....
Now, I do have a tube furnace and a quartz tube re: Garage Chemists SO3 Oleum synth, but I would LOVE to avoid that and have been using just 98% H2SO4
and Anhydrous Citric acid.
Oh how much hubris I had when I read 1899 Jerden and decided "pfffft I have vac distillation and a few fritted filters..... I need not a hydraulic
press as per EVERY 'real' scientist who ever made 1,3 acetonedicarboxylic acid...."
Well a bunch of wasted time (days and days and days and days) later I got a nice stainless steel press and away we went.
Again, typical of my syntheses I ran out of time and came back to the quenched classic (albeit modified to avoid oleum and use instead Conc. H2SO4)
reaction in the freezer and worked it up. Positive violet colour test with FeCl3 sol'n in H2O, so instead of taking the 15 minutes to do even a basic
melting point on it, away we went to waste a liter of acetic anhydride.
I'm fairly sure I eventually made Citraconic Anhydride because it all melted at basically 3 - 6 degrees C. But thats a crazy guess based on a
literature browse and not much else.
Onwards to the later Oxone and Cobalt & Tungstate catalyzed reactions (ragukumar2014; ordered those catalysts and awaiting their arrival), and
finally ending with a slight modification of a Chinese patent (CN103288629B) involving H2O2 oxidation with a catalytic amount of conc. H2SO4 and we
have a winner.
Prep scale for now as I don't want to waste any more Citric Acid, but a nice melting point of 134-136 C, and keeping it in a jar at room temp for even
a few hours had it decompose into a yellow mess and build pressure in the jar (A la' Acetone and CO2).
So.... short story long, I'd like to store the next batch as the anhydride and I wanted to know what Dr. Kaushal[sic] had to say about it which
spawned such rave reviews.
Cheers~!
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Quote: Originally posted by Johnny Windchimes  | Hey there,
I'd very much like to stabilize it (it decomposes quickly as you may know) for storage. I've been having some issues with the various methods (From
Kato 1966 & 1967 to Findlay 1950 and Casale 1987) and I think its basically my poor temperature control. That, and the ungodly "classic" synth via
H2SO4 and Citric acid that I could barely handle....
Now, I do have a tube furnace and a quartz tube re: Garage Chemists SO3 Oleum synth, but I would LOVE to avoid that and have been using just 98% H2SO4
and Anhydrous Citric acid.
Oh how much hubris I had when I read 1899 Jerden and decided "pfffft I have vac distillation and a few fritted filters..... I need not a hydraulic
press as per EVERY 'real' scientist who ever made 1,3 acetonedicarboxylic acid...."
Well a bunch of wasted time (days and days and days and days) later I got a nice stainless steel press and away we went.
Again, typical of my syntheses I ran out of time and came back to the quenched classic (albeit modified to avoid oleum and use instead Conc. H2SO4)
reaction in the freezer and worked it up. Positive violet colour test with FeCl3 sol'n in H2O, so instead of taking the 15 minutes to do even a basic
melting point on it, away we went to waste a liter of acetic anhydride.
I'm fairly sure I eventually made Citraconic Anhydride because it all melted at basically 3 - 6 degrees C. But thats a crazy guess based on a
literature browse and not much else.
Onwards to the later Oxone and Cobalt & Tungstate catalyzed reactions (ragukumar2014; ordered those catalysts and awaiting their arrival), and
finally ending with a slight modification of a Chinese patent (CN103288629B) involving H2O2 oxidation with a catalytic amount of conc. H2SO4 and we
have a winner.
Prep scale for now as I don't want to waste any more Citric Acid, but a nice melting point of 134-136 C, and keeping it in a jar at room temp for even
a few hours had it decompose into a yellow mess and build pressure in the jar (A la' Acetone and CO2).
So.... short story long, I'd like to store the next batch as the anhydride and I wanted to know what Dr. Kaushal[sic] had to say about it which
spawned such rave reviews.
Cheers~!
|
I feel its important to update this dead thread for anyone in the far future trying to make this stuff by saying that, no, the anhydride I thought I
formed was not indeed the anhydride of acetonedicarboxylic acid, as a FTIR I was able to semi-recently run at school unfortunately revealed.
Those Chinese patents don't work, or at least I could not get them to work, line for line, ACS quality reagent for reagent (ie no shortcuts were
taken).
That being said, I'm totally not the worlds best anything so if anyone else can make them work, please don't keep that info to yourselves!!
~Incredibly profound and/or wise quote goes here~
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Well JW, if it makes you feel any better I have tried most methods including the chinese patent with H2O2 and the only one that worked was conc
sulphuric and anhydrous ctric acid. But I couldn't get a decent yield of free acid from the crude product but I did discover that simply adding the
crude product to excess absolute ethanol and warming slowly to the 80-82 C for a few hours gave the diethyl ester in excellant yield (about 38% based
on citric acid) so I think this is the way to go since citric acid and conc sulphuric acid are cheap. I even bought a 500g jar once but once you have
opened it you have only hours to use it all and the yield of 1,3-diisonitrosoacetone was still very poor at <30%. In my opinion any preparation
which claims to use aqueous conditions in its preparation is total Bull S***!
There is a method that claims it is possible to convert diethyl or dimethyl citrate direct to the dialkyl acetone dicarboxylate ester. Given that
citric acid is a tribasic acid and that the acid groups are not identical I can see another minefield in this route too.
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Quote: Originally posted by Boffis  | Well JW, if it makes you feel any better I have tried most methods including the chinese patent with H2O2 and the only one that worked was conc
sulphuric and anhydrous ctric acid. But I couldn't get a decent yield of free acid from the crude product but I did discover that simply adding the
crude product to excess absolute ethanol and warming slowly to the 80-82 C for a few hours gave the diethyl ester in excellant yield (about 38% based
on citric acid) so I think this is the way to go since citric acid and conc sulphuric acid are cheap. I even bought a 500g jar once but once you have
opened it you have only hours to use it all and the yield of 1,3-diisonitrosoacetone was still very poor at <30%. In my opinion any preparation
which claims to use aqueous conditions in its preparation is total Bull S***!
There is a method that claims it is possible to convert diethyl or dimethyl citrate direct to the dialkyl acetone dicarboxylate ester. Given that
citric acid is a tribasic acid and that the acid groups are not identical I can see another minefield in this route too. |
Haha yes it does, lol, I've run the Reaxys gambit on this one myself.
Also, as long as we are discussing dead Acetonedicarboxylic Acid dreams....
Heres one from circa 1899 / 1900 that DOES work using Potassium Permanganate, but you need Mercury (sub)Sulfate to precipitate the Acetonedicarboxylic
acid before it over oxidizes. Not really my cup of tea....
CHIMIEORGANIQUE.—Sur 'oxydation manganique des acides citrique etmalique. Note de M. G. Denigès. 10 grams d'acide citrique ont été dissous
à chaud dans 20cc's d'eau; la solutionrefroidieversi5°aétéverséedansuneliqueurpréparéeendissolvant3 grams deMn04Kdans 100 cc's d'eau
chaude,refroidieensuiteài5°.» Lemélange,entouréd'eaufroide,change de teinteauboutdequelquesinstants,puisbrunitendégageantabondammentCO2. Pour
avoir un bon rendement, il faut éviter, dans cette réaction, que la températuredépasse 30° à 35° C
ORGANIC CHEMISTRY. — On manganic oxidation of citric and malic acids. Note from M. G. Denigès. 10 grams of citric acid was dissolved in 20cc's of
hot water; the solution cooled to 5° C was poured into a liquor prepared with 3 grams of MnO4K in 100 cc's of hot water, then cooled to 5° C The
mixture, surrounded by cold water, changes color after a few moments, then browns and releases abundant CO2. To have a good yield, it is necessary to
avoid, in this reaction, that the temperature exceeds 30° to 35° C.
(EDIT: I only Google translated a bit of his article, read the pages yourself for the Mercury part etc etc)
    
[Edited on 30-11-2020 by Johnny Windchimes]
~Incredibly profound and/or wise quote goes here~
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Or.... how About this bad boy?
In eine Losung von 14 g (0,1 Mol) Malonylchlorid[2] in 30 ml Chloroform (mittels P2P5 getrocknet) wurde bei einer Temperatur von -5 bis 0° im
Ganzen 3 Stunden lang (0,4 Mol/Std.) Keten eingeleitet. Die Reaktionsmasse wurde am Rückflusskiihler mit 25 ml absoluten Athanols versetzt und das
Losnngsmittel nach beendeter Reaktion durch Vakuumdestillation entfernt. Durch Fraktionierung des Rückstandes wurden 18,3 g (90 %)
Acetondicarbonsaure athylester vom Sdp. 105-1100 /1 mm gewonnen. Eine Zur Analyse destillierte Probe be sass einen Sdp. von 92º/0.5 mm,?D20 1,4420,
d420 1,113. Das Semicarbazon derselben wies den Smp. 96° auf; die Mischschmelzpunktbestimmung mit einer authentischen Probe (Smp. 96°) ergab keine
Depression. Die angefiihrten Werte entsprechen den Literaturangaben[3]translation----------
-In a solution of 14 g (0.1 mol) of malonyl chloride [2] in 30 ml of chloroform (dried using P2P5) was ketene at a temperature of -5 to 0 ° for 3
hours (0.4 mol / h) initiated. 25 ml of absolute ethanol were added to the reflux condenser, and the solvent was removed by vacuum distillation after
the reaction had ended. Fractionation of the residue gave 18.3 g (90%) of acetone dicarboxylic acid, ethyl ester, bp 105-1100 / 1 mm. A sample
distilled for analysis had a bp of 92 ° / 0.5 mm, ?D20 1.4420, d420 1.113. The semicarbazone thereof had a mp of 96 °; the mixed melting point
determination with an authentic sample (mp. 96 °) showed no depression. The values ??given correspond to the literature data [3]
I will also add that yeah....ketene....bad....deadly....dangerous....etc....
You'll also have to forgive the previous French and German excerpts I posted; they reek of Google translate!
Source for that and even MORE obscure acetonedicarboxylic acid refs in the attached ref doc, and I threw in the Reaxys doc too because, why not?
Attachment: ADA Reaxys - Copy.pdf (1.2MB)
This file has been downloaded 310 times
Attachment: Many Pathways to Acetonedicarboxylic - Copy.pdf (2.6MB)
This file has been downloaded 589 times
~Incredibly profound and/or wise quote goes here~
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: |
R. Kaushall, J. of Indian Chem. Soc., 17, 138 (1940).
|
It is abstracted in British Chemical Abstracts, available online.
However, fortunately, needed article is also available. To save the time - the article in attachment.
Attachment: Kaushall.pdf (265kB)
This file has been downloaded 328 times
Слава Україні !
Героям слава !
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Quote: Originally posted by kmno4  | | Quote: |
R. Kaushall, J. of Indian Chem. Soc., 17, 138 (1940).
|
It is abstracted in British Chemical Abstracts, available online.
However, fortunately, needed article is also available. To save the time - the article in attachment. |
WHERE WERE YOU 9 MONTHS AGO!?!
THANKS!!  
~Incredibly profound and/or wise quote goes here~
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I am still around, but I am not sure if the article was available online 9 months ago... Entire volume 17 is available, but 16 or 18 vols. are not
 . .
Слава Україні !
Героям слава !
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
@ Boffis:
If its esters you seek, I'm sure you're aquianted with these fine docs:
It's doable, but looks tedious as all hell IMO....
Order of reading should be Green, Hirota, Walton I think....
Attachment: GREEN DOWEX - turhanen2019 - Copy.pdf (1.5MB)
This file has been downloaded 305 times
Attachment: Asym Monomethyl Citrate - hirota1980 - Copy.pdf (5.7MB)
This file has been downloaded 337 times
Attachment: US2848480 - Walton Patent - Copy.pdf (569kB)
This file has been downloaded 291 times
~Incredibly profound and/or wise quote goes here~
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
*File was too big to include above*
Attachment: Citric Acid Esters - Copy.pdf (486kB)
This file has been downloaded 281 times
~Incredibly profound and/or wise quote goes here~
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Exotic reagent.
One might jump to the conclusion, that the Acetone Dicarboxylic Acid Anhydride, might be destined for an exotic product.
Like being reacted with an alkyl alcohol, to form the half ester. Followed by a condensation that produces a Tropanone.
A challenge.
Now, I wouldn't take on the challenge, because the reduced Tropanone. Or Tropanol, would have two centers of assymetry.
Meaning that, in the final product, four isomers would be present. Three of them, of doubtful physiological
activity.
Though perhaps I am mistaken?
[Edited on 6-12-2020 by zed]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
IIRC you can also use periodic acid to oxidize citric acid instead of oleum but the conditions are hard to get right. Jackson attached mentions
production of acetaldehyde by oxidation of lactic acid for example but acetaldehyde itself degrades under the rxn conditions.
Attachment: jackson2011.pdf (3.4MB)
This file has been downloaded 295 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
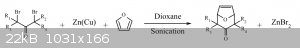
Will if tropinone, or some other analog, is what you’re after I always thought a zn(cu) couple with 1,3-dichloroacetone (or dibromo) and
N-methylpyrrole would be an option. Then a hydrogenation of the double bond followed by a Claisen condensation with dialkyl carbonate. If you were so
inclined. Or do the hydrogenation after the Claisen condensation. That might be a better option
[Edited on 8-12-2020 by Opylation]
|
|
|