Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
copper sulfate using electrolysis
I made a lot of mistakes in my life obviously :~
Like throwing 500g copper chloride just because I stored it in a plastic container had lead oxide from car battery in it and cuz I'm stupid I didn't
remember so I didn't like ANY 0% toxic metals in my pretty CuCl2 :/
yep even though I cleaned the container properly but without acid bath
So I started from the beginning
I produced a good amount of copper sulfate for some copper experiments I want to try in the very near future. What I've been doing lately is running
the 12v from a battery charger right into a beaker containing 30% H2SO4 and copper electrodes as you can see  my copper source is wires always cuz I'm sure 1000% pure copper with no Cd, As, Hg, Tl... my copper source is wires always cuz I'm sure 1000% pure copper with no Cd, As, Hg, Tl...
   

|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Why do you thing that in everything is arsenic, mercury, thallium, cadmium, lead etc?
Aren't you Tahalium? Your panic fear from heavy metal poisoning and "cuz" in your text really strongly reminds me him.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Lead acid batteries contain lead and antimony with small amounts of barium sulfate
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
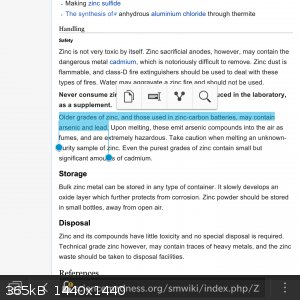
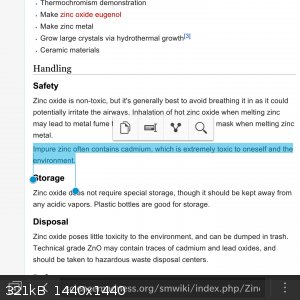
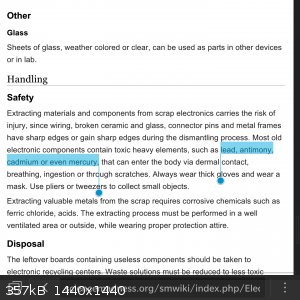
Ahah I'm sorry, the old tahallium can't come to the phone right now, why? Cause he's dead!
YOU'RE ASKING ME WHY?
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Copper Sulfate
I wouldn't make CuSO4. It's too easily
available as root killer. When my nephews
were growing up I showed them how to
grow crystals with it. 1 nephew used it
for electroplating carbon rods as a science
project.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Lucky! Here in Tunisia I can't buy potassium carbonate and they think EVERY ammonium salt: chloride sulfate or even phosphate is explosive as f just
cuz there's ammonium in it  fml fml
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
best way to get 100% pure CuSO4 is to react H2O2 + H2SO4 and Cu but if you got lot of time i mean like a month, leaving Cu in H2SO4 in open air to
react with O2 will make CuSO4.
"A mind is a terrible thing to lose"-Meisner
|
|
|
B(a)P
International Hazard
    
Posts: 1116
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by Fluorite  |
I produced a good amount of copper sulfate for some copper experiments I want to try in the very near future. |
Nice experiment, what are you planning to do with the copper sulfate?
Quote: Originally posted by Bedlasky  |
Aren't you Tahalium? Your panic fear from heavy metal poisoning and "cuz" in your text really strongly reminds me him. |
I think Fluorite is trying for a new start after learning from his mistakes and some advice received on this forum.
If the OP does not have access to CuSO4 I doubt they can get their hands on H2O2?
|
|
|