vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Praseodymium(III)-potassium sulfate - PrK3(SO4)3
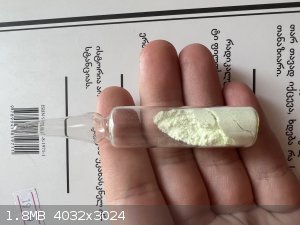
Hello! I had just 0.5-1 g praseodymium carbonate, so I made PrK3(SO4)3*H2O. it's easy to make it, just mix sulfates solutions. it has low solubility,
so it precipitates instantly. not as green as other Pr compounds, but it has a weak greenish color.
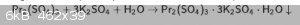


|
|
|
unionised
International Hazard
    
Posts: 5105
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Pretty.
Does it fluoresce?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4280
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nice! I wonder how you could mix the solutions slowly enough to get crystals.....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I don't know ill try uv in this days.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4280
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
May I suggest that you try the sulphamate trick to get crystals of this?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
andyloris
Harmless

Posts: 49
Registered: 22-9-2022
Location: France
Member Is Offline
Mood: Lazy
|
|
What is the sulphamate trick
|
|
|
j_sum1
Administrator
       
Posts: 6232
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
From a recent thread…
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
I hadn't heard of it either but it seems like a really cool idea. I might attempt it myself sometime soon.
|
|
|