SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Stellate Crystals - A Morphologic Study
I've observed that some salts which are inclined to super-saturate when evaporated from solution have a tendency to form unique star-like ('stellate')
crystals consisting of one or more aggregates of needle-like crystals that radiate outwards from a central node.
In this post I'll explain my understanding of the stellate crystals' morphology and appearance.

Stellate crystals formed by zinc acetate (one of its several possible crystal structures). Click to view full res. image.
The stellate crystals' microscopic structure results in unusual optical properties, creating a lenticular surface that resembles that of the machined metal finishes produced by lathe facing operations.
 - - - - 
A set of light renders I made (left) and a picture of some 'engine-turned' metal surfaces (right) consisting of singular spiraling grooves.
The cause of this appearance can be understood better with the light renders I made (see above), which show the structures' topology and how light
interacts with it. The 'radial grooves' renders are a fairly accurate representation of the stellate crystals' microscopic structure.
For comparison, the 'spiral grooves' renders depict the surface produced by an (idealized) lathe turning operation. The 'concentric grooves' renders
are included to show their near-identical appearance to the spiral grooves.
Notably, the light reflecting off the stellate crystals forms a 'bowtie' shape that extends perpendicular to the light source while the spiral lathe
surface forms a parallel bowtie. All three structures are otherwise nearly indistinguishable on a macroscopic scale.
 - - - - 
A 'mononuclear' stellate crystal formed by sodium acetate (left) and a 'polynuclear' stellate crystal produced by sodium citrate (right).
Stellate crystals can have one or more nuclei. The number of nucleation centers depends on the rate of nuclei emergence ('primary nucleation') and the
rate of crystal propagation ('secondary nucleation'), which in turn are determined by the rate of solvent evaporation and the enthalpy of
crystallization, and perhaps other factors.
-
In the following diagram, I've annotated the individual nuclei and cell boundaries of a polynuclear sodium citrate crystal that I grew and
photographed last week.
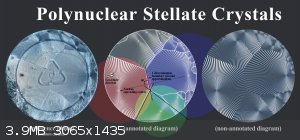
Click to view full res. image.
Although each nucleus initially forms a circular stellate crystal (due to their roughly equal rate of propagation in all directions), the edges of
each cell are usually a straight line.
This is because two circles expanding at equal rates will terminate to form a straight bisectual line, due to some or other geometric principle.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Growth of Stellate Crystals and Misc. Notes
The growth of stellate crystals by seeding of a super-saturated solution of sodium acetate can be seen in this video I recorded or another by Wayne Schmidt on youtube.

Wavelite mineral photo posted by Wikipedia user Parent Géry.
Stellate crystals can also occur naturally, for instance as the aluminium hydroxyphosphate mineral 'Wavelite' of the formula
'Al₃(PO₄)₂(OH)₃·5H₂O'.
 
Stellate crystals of sodium acetate (left) and magnesium acetate (right).
As shown in the above two photos, the individual crystals making up a stellate crystal aren't necessarily microscopic, and under certain growth
conditions can be quite large, although they forfeit their bizarre optical properties.
-
I'm not sure the observations I've made in this post are useful or particularly interesting, but the zinc acetate crystals in the previous post blew my mind the first time I saw them and they're something I've wanted to write about for some time
after I'd figured out how they form and why they look the way they do.
Tags: Lenticular crystals, topography of sodium acetate crystallized from a super-saturated solution, optical properties of
stellate crystals
[Edited on 8/22/2023 by SnailsAttack]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I remember watching ascorbic acid crystallizing from isopropyl alcohol through a microscope. Crystallization would start at several points, and
propagate outwards as a wave. Once it got so big, the speed of the outward propagation would drop sharply, and finally stop. Once that happened,
nucleation sites on the stalled front would spawn new semicircular waves and the cycle would repeat. Some very interesting dynamics, maybe some sort
of critical slowing-down.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by mayko  | | I remember watching ascorbic acid crystallizing from isopropyl alcohol through a microscope. Crystallization would start at several points, and
propagate outwards as a wave. Once it got so big, the speed of the outward propagation would drop sharply, and finally stop. Once that happened,
nucleation sites on the stalled front would spawn new semicircular waves and the cycle would repeat. Some very interesting dynamics, maybe some sort
of critical slowing-down. |
I know exactly what you're talking about, i think. Did it look sort of like this sketch? My sodium citrate did it one time but I forgot to take a
picture.
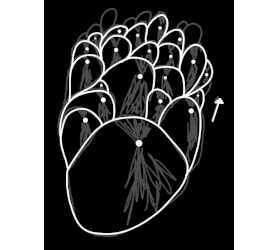
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
I learned that the 'engine-turned' (or 'radial-brushed') surface appearance is also known as radial anisotropy, which fetches a lot of search results pertaining to its use in digital rendering.
|
|
|