vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Help with calculations for copper plating solution
I found this paper about making a copper plating solution and thought I'd give it a shot. Please keep in mind I have no formal education in chemistry.
I have a few questions about the preparation of the solution.
Information:
The paper lists the following ingredients:

In the conclusion, the authors recommend the following additives:
500 ppm PEG
5 ppm SPS
30 ppm JGB
PEG:
The PEG I have is labeled as PEG 400. The paper doesn't say anything about this. I assume the paper calculates as if it was pure PEG, where mine is
400 g / mol? Could someone guide me on how to calculate the quantity needed to achieve 500 ppm?
Cl-:
For chlorine, I am considering hydrochloric acid. The concentration of the acid I have is 30%, and I would need 1.4 mM of chlorine. Since I need only
a very small amount, It might be best to dilute the concentrated acid. I do have a very precise scale, but weighing out 0.05 gram seems a bit
ridiculous.
Results:
Here is what I've calculated so far:
| Code: |
Chemical Molar mass Required Result
CuSO4 5H2O 159.609 g/mol 0.79 M 126 grams
H2SO4 98.079 g/mol 1.02 M 100 grams
HCl 36.460 g/mol 1.40 mM 0.05 grams
SPS 5 ppm 5 milligrams
JGB 30 ppm 30 milligrams
PEG400 500 ppm
|
This is for a 1-liter solution, with the remainder being deionized water. I'm aware of the acid-to-water addition protocol.
Power supply:
The paper specifies a particular waveform. For now, I would like to ignore this and just use a current-limited DC power supply.
- Paper: https://www.mdpi.com/2076-3417/8/11/2135
|
|
|
knowledgevschaos
Harmless

Posts: 29
Registered: 9-8-2023
Location: Sci-Hub and the hardware store
Member Is Offline
Mood: Hungry for information
|
|
Keep in mind I am also very inexperienced in electroplating, but here goes:
Concentrations in ppm are usually done by mass. My guess is that you should not worry about the molecular weight of your PEG, but instead you should
just measure 100 - 500 mg of PEG per kg of solution. I'm not sure if the molecular weight of your PEG would interfere with the process if it was the
wrong weight, but given that it doesn't seem to specify a weight in the paper, I think it should work.
The paper also says chloride instead of chlorine, so you might be able to get away with using table salt or another chloride. I don't know if the
sodium would interfere with the electroplating however, so hydrochloric acid is probably the safest option.
I hope this helps, but it's just my speculation, so take it with a grain of salt.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
If you dont have a scale that can do a certain small amount, do it in two, three or more steps. For example three steps of ten, first dilute your
concentrated acid; 5 grams in a kilo. Then dilute this ten times. Now for your final solution you can take 100 milliliters of this instead of 0.05
grams of the concentrated acid.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
@Tsjerk, I'm planning on using that method, although I have a very nice scale, this seems more practical.
@knowledgevschaos, I'm not sure about this, the different numbers seem to indicate the molar mass of the PEG. I think 500 ppm of PEG400 is completely
different to 500 ppm of PEG 4000. But this is where I lack the knowledge.
Can someone verify my calculations? The result's column is what I have calculated. And I'm not sure if it's correct.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
One 'drop' of hydrochloric acid is close to 0.05ml,
if (as I suspect) only approximate ratios are required.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
The consentration of your h2so4 and hcl are not listed. This will have a major effect on the mol/weight ratios
PEG400 sseams a little short. Ive never tried it. It but had good results between 1500-3500.
Basicly that number is an indication of the polymer chain length
So the
copper sulfate - the Cu source
Sulfuric acid - increase the mobility of all the ions.
cl- - increases anode erosion to help keep the Cu+ levels up and lower reaction potential
SPS ??
JGB ??
PEG - minimize current density gradients and increase throwing power.
I highly recommend you review data logging for these experiments. Leanwhat values to record and analyze will help you troubleshoot issues.
There is a trick you can do to get very good data cant remimber what its called.
Google......
A "Hull cell" is a cheap and easy way to measure performance
"You can't do that" - challenge accepted
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
@vanBassum: You're right, 400 indicates the average molecular weight of the PEG. Since the recipe doesn't specify the MW it probably isn't super
critical. You can assume you have 100% PEG unless the label indicates something else.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Hello,
I have tried this with a 100ml batch, and so far I get a decent coating of copper. It hatches well to the material and seems to be deposited evenly.
However, the finish is dull instead of shiny. After some reading, I think PEG400 is too low to get a good result and I should use a higher PEG rating.
To answer a couple of questions:
My H2SO4 is concentrated 98%
My HCl is 36%, although I haven't verified this. The bottle is about a year old.
PEG stands for poly(ethylene glycol)
- Used as suppressor
SPS stands for Bis-(3-sulfopropyl) Disulfide
- Used as accelerator
JGB stands for Janus Green B
- Used as leveler
Calculations:
Prepare scaled down solutions:
Make 1% SPS solution:
1000 mg SPS + 9000 mg water = 10000mg of 1% solution.
Make 1% JGB solution:
1000 mg JGB + 9000 mg water = 10000mg of 1% solution.
Make 1% PEG solution:
1000 mg PEG + 9000 mg water = 10000mg of 1% solution.
Make 1% HCl solution:
100 mg HCl @ 36% + 3500 mg water = 3600 mg of 1% solution.
1% HCl => 36.46 / 0.01 = 3646 g/mol
Prepare electrolyte 1 kilo (more or less 1 liter)
Add CuSO4 5H2O:
CuSO4 5H2O = 159.609 g/mol
0.79M * 159.609 g/mol = 126 gram
Add H2SO4:
H2SO4 = 98.079 g/mol (Mine is 98%)
1.02M * 98.079 g/mol = 100 gram
Add HCl:
HCl @ 1% = 3646 g/mol
0.0014M * 3646 g/mol = 5.1044 gram
Add PEG:
500ppm of PEG = 500 mg total
500mg @ 1% = 50 gram
Add SPS:
5ppm of SPS = 5 mg total
5mg @ 1% = 0.5 gram
Add JGB:
30ppm of JGB = 30 mg total
30mg @ 1% = 3 gram
[Edited on 26-10-2023 by vanBassum]
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
The solution to use depends on what you are doing. Some baths are best for building thick layers of copper (electroforming) (usually a rough surface
results), some are better for a smooth (usually thin coating). Some baths will plate onto steel or other bases, some will not.
Industrially sometimes time is money, sometimes electrical efficiency is more important.
The SPS and JGB could be used as brighteners (smoother finish).
Some additives give a better throw (plating inside low areas or holes).
Much depends on the current density (amps per square foot), cell voltage or temperature.
Sometimes agitation helps.
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Also keep tract of available copper.
Im assuming your using a copper anode.
Take accurate weight measurements of your item befire and after plating.
As the process evolves, your solutiin will change. For accurate analysis try to keep tract of these changes.
Try to record
Total current
Applied voltage
Before and after weights of item and anode
Distance copper travels from anode to different points on the item
This will give you a good baseline for judging hownyour solution is performing
"You can't do that" - challenge accepted
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Any good plating procedure should specify the electrical parameters as well as the chemical. You need to control parameters like temperature, current
density and anode/cathode ratios as well.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Thanks for the tips so far.
First, I ordered PEG 1500 as by the recommendations of @Rainwater. I have seen this noticed on some places online as well. It will take a bit to
arrive. Next up, the hull cell, also mentioned by @Rainwater, seems like a nice piece of equipment. I plan on using my 3D printer to make one and coat
the print in epoxy resin to make it water tight. Or I just buy one online, but for a first test this seems fine.
I like the idea of tracking the different parameters, like:
- Voltage
- Current
- Temperature
- Weight of anode
- Weight of cathode
- The distance between electrodes, although this static as defined by the hull cell.
I'm considering building a logger for voltage, current and temperature. I think I have everything I need at home.
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Peg 3500 is sold at pharmacys as stool softener
A hull cell is simply an electrode arrangement.
You can buy a jig for 20-80 bucks
or just ensure your electrodes are secured in place at the proper distance and angles
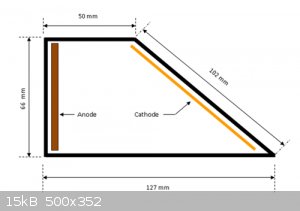
image source
For 3d printed parts you need to ensure compatability of your print
Pla and abs will dissolve in your proposed solution.
"You can't do that" - challenge accepted
|
|
|