falingunit
Harmless

Posts: 4
Registered: 20-11-2023
Member Is Offline
|
|
Alternative catalyst for oxidation of SO2 to SO3 (first post here btw)
I'm planning on making sulfuric acid through the Contact process. Unfortunately I do not have access to Vanadium Pentoxide. I might be able to ozone
though. If anyone knows any other catalyst (other than Pt) it will be of great help.
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 151
Registered: 12-7-2018
Member Is Offline
|
|
Winkler wrote Ironoxyde works to but only with 75% yield.
|
|
|
Parakeet
Hazard to Self
 
Posts: 70
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Nice to meet you!
Actually if you search this forum, you can find many discussions about this topic.
Regarding your questoin, I think this post provides an interesting information.
https://www.sciencemadness.org/whisper/viewthread.php?tid=28...
|
|
|
unionised
International Hazard
    
Posts: 5105
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
This might be interesting.
https://pubs.acs.org/doi/10.1021/ed076p958
|
|
|
Texium
|
Thread Moved
24-11-2023 at 13:43 |
falingunit
Harmless

Posts: 4
Registered: 20-11-2023
Member Is Offline
|
|
I might give iron oxide a go. Using pure oxygen instead of atmospheric oxygen should increase the conversion rates significantly I think. I actually
have no idea how to can setup a catalyst bed of iron oxide. I can use a gas stove to heat it up but it will be hard to control the temperature. So,
how can I setup an iron oxide catalyst bed?
|
|
|
Admagistr
Hazard to Others
  
Posts: 350
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by falingunit  | | I might give iron oxide a go. Using pure oxygen instead of atmospheric oxygen should increase the conversion rates significantly I think. I actually
have no idea how to can setup a catalyst bed of iron oxide. I can use a gas stove to heat it up but it will be hard to control the temperature. So,
how can I setup an iron oxide catalyst bed? |
You could do it with glass wool, which you saturate with a concentrated solution of Fe(NO3)3 and then anneal somewhere outside, NO2 will escape, on
the wool Fe2O3 will be excluded.Fe(NO3)3 is easily decomposed by heat, much better than sulphate.
[Edited on 25-11-2023 by Admagistr]
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 151
Registered: 12-7-2018
Member Is Offline
|
|
Early Catalysts for SO2 to Sulfuric acid are: quartz pieces, porcelain,brick.Reaction worked but was to slow.
bettwer was pumic stone soaked in Platinchloride.
Working with concentrated SO2 is better than with strong deluted one with air.
Yields are higher if you use pure o2.
Chrome and Copperoxide works to but also not so desireable.
[Edited on 25-11-2023 by Alkoholvergiftung]
|
|
|
falingunit
Harmless

Posts: 4
Registered: 20-11-2023
Member Is Offline
|
|

(from the thread Parakeet linked)
What does Karbonyl Iront IG mean? It seems to have a higher yield.
|
|
|
barbs09
Hazard to Others
  
Posts: 113
Registered: 22-1-2009
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Fun old articles on small scale preparation of H2SO4 using iron oxide catalyst 
Cheers,
AB
Attachment: Dangerous acids made a home.pdf (530kB)
This file has been downloaded 115 times
Attachment: Learn about Sulphuric acid.pdf (324kB)
This file has been downloaded 90 times
[Edited on 1-12-2023 by barbs09]
|
|
|
falingunit
Harmless

Posts: 4
Registered: 20-11-2023
Member Is Offline
|
|
Here's my idea:
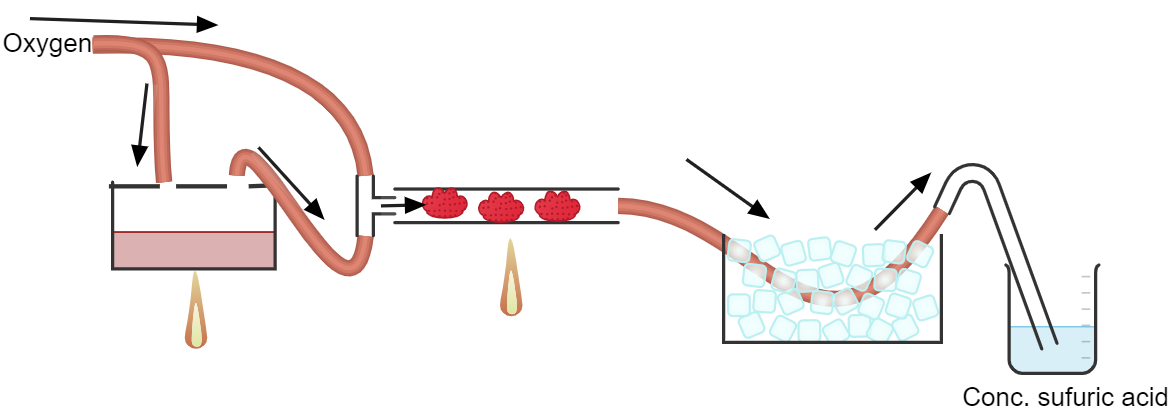
Only the tubing connected to the o2 supply are silicone. All other tubing have to be stainless steel. The sulfur burner would be an empty paint can,
and the catalyst tubing would be made of stainless steel.
The O2 supply is from a cylinder an not a generator.
The gas coming out of the catalyst tube will be at 600C. I don't think bubbling it straight into water is a good idea. Especially considering that
sulfur trioxide itself already reacts exothermically with water. I probably have to cool it down somehow before dissolving in it conc. sulfuric acid
to get oleum and then dilute it.
I'm still not sure how to make the catalyst. How can I get Iron(III) oxide nanoparticles (if they are even possible) and how can I disperse it in the
catalyst tube to maximize efficiency.
[Edited on 2-12-2023 by falingunit]
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 151
Registered: 12-7-2018
Member Is Offline
|
|
http://dingler.bbaw.de/articles/ar113036.html
Alternative way from 1848 for the manufacture of Sulfuric acid without lead chambers. It is in german.
If you watch the drawings it seams they had an working factory with this methode. Pumic stone with 1% MnO2.
|
|
|
EF2000
Hazard to Others
  
Posts: 113
Registered: 10-5-2023
Location: The Steppes, now trapped in the forest zone
Member Is Offline
Mood: wrooom
|
|
German Wikipedia article on iron pentacarbonyl says (in translation):
| Quote: |
Iron pentacarbonyl is used to produce semi-transparent iron oxide pigments with very high chemical purity. Combustion takes place in an excess of
atmospheric oxygen at temperatures between 580 °C and 800 °C. This results in orange to red, amorphous products with small grain sizes between 10
and 20 nm. |
So, Karbonylrot is something like nano-particles of red iron oxide (rot=red), produced by burning pentacarbonyl (Karbonyl, called "carbonyl" in modern
German)
Wroom wroom
|
|
|