| Pages:
1
2 |
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Synthesis of terephthalic acid from a PET water bottle
Hello people,
I am back with another episode of chemical recycling, and our victim today will be again a waste water bottle. Let's find out!
The last episode was about the preparation of benzene by pyrolysis of waste PET water bottles, this time instead, from the same waste material we synthesize terephthalic acid.
The reaction involved is simple acid hydrolysis of PET, polyethylene terephthalate, into its precursors acid and glycol. The latter will not be
recovered though.
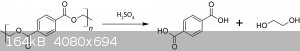
Procedure
A water bottle is removed from cap, seal, label, top, and bottom. The body is cut into pieces; 20 grams of that are introduced into a large beaker.
Apart, 20 ml of distilled water is brought to 150 ml with concentrated sulfuric acid. The solution gets very hot. While still hot, the acid solution
is poured into the PET pieces and stirred slowly. PET flakes soften, then melt and dissolve completely. Once done, the reaction mixture is kept under
stirring for an additional 1.5 hours, then an equal amount of water is added - 150 ml: a grey suspension forms, mixture heats up. Once cool, an
additional 1 liter of water is added, and the suspension gravity filtered. The residue is oven-dried, then dissolved with 250 ml of aqueous KOH. The
solution is gravity filtered, the filtrate is slowly added of 100 ml HCl 37%: a white suspension forms. That is stirred for a while, then vacuum
filtered, washed with methanol, and oven-dried.
5.83 g of white solid is left, 29.4% of the initial PET mass.
m.p: > 300 Celsius

Discussion
I think this experiment is wonderful, and considered one of my best chemical recycling examples. From total trash, a useful chemical can be obtained,
which in turn can be converted to something else. The yield can be improved, I think, by exploring different conditions, as well as cost using NaOH
rather than KOH, a minimum amount of HCl, ethanol in place of methanol.
I could measure the proton NMR spectrum of product in d-DMSO and it looked very pure !
As usual, I link my YT video, both preparation and analysis.
Thanks for attention
See you next time, with the esterification of that acid,
palico
[Edited on 2-2-2024 by palico]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Well done! Nice experiment! Yes, much cleaner than pyrolysis. Costs could be perhaps reduced by avoiding H2SO4 and hydrolyzing directly in sol. of
NaOH? I wonder whether less of hydroxide as well acid cannot be sufficient for only 20 g of PET?
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fery  | | Well done! Nice experiment! Yes, much cleaner than pyrolysis. Costs could be perhaps reduced by avoiding H2SO4 and hydrolyzing directly in sol. of
NaOH? I wonder whether less of hydroxide as well acid cannot be sufficient for only 20 g of PET? |
PET hydrolysis in NaOH, requires long reaction time and high temperature.
Yes, use a minimum amount of HCl and NaOH is better to reduce production cost.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
It is very unusual way of hydrolysing of PET.
A method for very rich and very mad madscientists, I would say.
It apparently works, but it is hard to call it useful, when someone converts 10 dollars into 10 cents.
PET is easily hydrolysed by > 20% NaOH, r.t. is good, heating speeds up the reaction, but it is optional. Disodium TPA salt is obtained in
solution. Next, TPA can be precipitated with some acid, for example citric one, to reduce costs. NaOH method is described in many places, also at SM,
and I am not going to bother readers with commonly known things.
Слава Україні !
Героям слава !
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
There’s nothing wrong with demonstrating alternative methods. It may be easy to forget that outside of the EU, conc. sulfuric acid is readily
available and cheap in most of the world.
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | It is very unusual way of hydrolysing of PET.
A method for very rich and very mad madscientists, I would say.
It apparently works, but it is hard to call it useful, when someone converts 10 dollars into 10 cents.
PET is easily hydrolysed by > 20% NaOH, r.t. is good, heating speeds up the reaction, but it is optional. Disodium TPA salt is obtained in
solution. Next, TPA can be precipitated with some acid, for example citric one, to reduce costs. NaOH method is described in many places, also at SM,
and I am not going to bother readers with commonly known things. |
Have you ever tried such reaction for real ? Are you sure you got TPA ? Because such procedure does not eliminate oligomers, I think. Mine, as you can
see from proton NMR, give very pure TPA.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Texium  | | There’s nothing wrong with demonstrating alternative methods. It may be easy to forget that outside of the EU, conc. sulfuric acid is readily
available and cheap in most of the world. |
As usually, you have something hopeless to say. You start getting on my nerves.
Слава Україні !
Героям слава !
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by kmno4  | Quote: Originally posted by Texium  | | There’s nothing wrong with demonstrating alternative methods. It may be easy to forget that outside of the EU, conc. sulfuric acid is readily
available and cheap in most of the world. |
As usually, you have something hopeless to say. You start getting on my nerves. |
Oh really? Good. At least
this time you actually say it instead of just deleting the post I called out like you usually do.
|
|
|
Boffis
International Hazard
    
Posts: 1837
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Now now ladies, please put those handbags away!
This is an interesting method for the hydrolysis of PET resin but as a preparatory method it seem to have limited use. As kmno4 says the large amounts
of chemicals, no matter how cheap and available, for a 34% yield based on the assumption that the PET is 100% pure, is not great. Does the original
poster have any idea where the other 66% of the terephthalic acid has gone?
The alkali hydrolysis method carried out carefully yields 75-90% and if the liquor is recycled this can be raised to 95% easily. Only NaOH, water,
dilute HCl and ethylene glycol are required and the latter is not essential but does reduce the reaction time by allowing higher temperatures.
|
|
|
bnull
Hazard to Others
  
Posts: 150
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
In the discarded solution. If the PET didn't hydrolise completely, there may have been oligomers terminated by glycol. Ethanol reacts with sulfuric
acid to form ethyl sulfate as long as the temperature is below 140°C; I suppose the same happened to the oligomers, which reacted with the acid
(because there was too much of it anyway), forming water-soluble compounds.
For each terephthalic acid that leaves, there are at most two glycol terminations free to be attacked. With two terminations forming sulfate, the
oligomer is much more soluble. After the addition of water, the terephthalic acid precipitated, but not the oligomer sulfate.
@palico, did you keep the solution? If you kept it, you could try to precipitate the partially reacted stuff (supposing my idea makes sense, of
course) by using NaOH to neutralize the acid. Maybe it works.
Or maybe I'm dead wrong.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@Boffis: I do not know what is your 34% yield. We do not know yield because the molecular weight of PET is not known, and of course it is a mixture.
5.84 g of TPA are the 29.4% of 20 g of PET.
bnull: your recipe require heating for long time at high temperature, energy cost today a lot, and also glycol. The acid hydrolysis instead can be
realized with household chemicals. Anyway, have ever measuread at least proton NMR of your TPA got from basic glycol procedure ?
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Very interesting procedure! I add it to list of my future experiments. I appreciate your detailed report, unlike kmno4.
Quote: Originally posted by palico  | | @Boffis: I do not know what is your 34% yield. We do not know yield because the molecular weight of PET is not known, and of course it is a mixture.
5.84 g of TPA are the 29.4% of 20 g of PET. |
Just consider molar mass of monomer. Monomer contains 166/210 = 79,05 % of terephtalic acid. That's 0,7905*20 = 15,81 g of terephtalic acid in 20 g of
PET. You isolated 5,84 g, so 5,84/15,81 = 36,94 %.
|
|
|
bnull
Hazard to Others
  
Posts: 150
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
Quote: Originally posted by palico  | | bnull: your recipe require heating for long time at high temperature, energy cost today a lot, and also glycol. |
My recipe? What recipe? It was @kmno4 who first suggested the alkaline hydrolysis, not me. For fleck's sake... I just offered an explanation as to why
the yield was not even close to what one obtains from the NaOH procedure.
I'll ask again: did you discard the acidic solution? Would you care to verify, if you still have it, what happens when you neutralise it with a
hydroxide? I'm very interested to know if something precipitates out of the solution or the solution gets cloudy or a gel forms or whatever the
flaming hell happens, if something at all. Yes, I'm that curious.
If, and only if, a suspicious crud sinks to the bottom or rises to the top (I don't know what is the density of all
the oligomers, you see), there will be the rest of the TPA, still attached to at least two glycol terminations.
And yes, I've read some very interesting papers about hydrolysis of PET in concentrated H2SO4, all of them involving heating the
mixture. The yield was above 95%.
[Edited on 7-2-2024 by bnull]
And also yes, your idea is quite interesting. I should have mentioned it before. I appreciate alternative ways to perform syntheses, or decompositions
as it seems to be the present instance.
[Edited on 7-2-2024 by bnull]
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@Bedlasky: ah, I see yield is more than conversion, good. Anyway, I do not believe the basic procedure got 95% yield, I want to see proton NMR first.
@bnull: the mixture is heated in my experiment. Mixing water and sulfuric acid makes the solution get very hot, and I poured while it still was. I
think longer reaction time give higher yield.
|
|
|
bnull
Hazard to Others
  
Posts: 150
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
What was the temperature?
With continous heating, I suppose.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
Boffis
International Hazard
    
Posts: 1837
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bedlasky  | Very interesting procedure! I add it to list of my future experiments. I appreciate your detailed report, unlike kmno4.
Quote: Originally posted by palico  | | @Boffis: I do not know what is your 34% yield. We do not know yield because the molecular weight of PET is not known, and of course it is a mixture.
5.84 g of TPA are the 29.4% of 20 g of PET. |
Just consider molar mass of monomer. Monomer contains 166/210 = 79,05 % of terephtalic acid. That's 0,7905*20 = 15,81 g of terephtalic acid in 20 g of
PET. You isolated 5,84 g, so 5,84/15,81 = 36,94 %. |
Hi Bedlaski/palico. No! its not the monomer you have to consider, its the monomer less one mole of water (lost through the ester linkage). So the MW
of the monomer is 210.18 but the MW of the simplest monomeric unit is only 192.17. so in the first case the theoretical yield is 15.81g of
Terephthalic acid while in the second case it is 17.29g. thus palico's yield of 5.84g is 5.84/17.29 x 100 = 33.77% not 36%.
However, 2% difference hardly negates the validity of my question. And I suspect bnull's answer is close to the truth. Terephthalic acid is sparingly
soluble even in fairly strong sulphuric acid so I suspect that your hydrolysis was incomplete.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
It seems, that above insane procedure is taken from patent literature (US3952053 or US4355175), with totally changed amounts of reagents. The original
proportions PET/H2SO4 from the patents are much more "user friendy". This is info is for lazy Americans from the most of the world, but there is no
need to thank.
The NaOH method is still preferred, because less steps are needed, no need to use H2SO4/HCl/HNO3/H3PO4 at all, recovery of ethylene glycol is much
simpler (if someone needs).
Слава Україні !
Героям слава !
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@bnull: Yes, looks a contradiction, man I am not native english speaker. The r. mixture is naturally heated by the sulfuric acid solution. That's all,
no additional hotplate heating applied. No, I did not measure temperature.
@Boffis: I continue to trust more conversion than yield in this case. Yes, conversion or yield, of course reaction did not go 100% completion. Next
time, I will try longer reaction time.
@kmno4: I do not remember reference for this procedure. I did not use any nitric or phosphoric acid.
|
|
|
bnull
Hazard to Others
  
Posts: 150
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
Before the this thread degenerates into a flame war (I hope not), I want to ask some questions about the procedure and maybe even suggest some ideas.
Why? Because I'm f***ing curious.
@palico: First, I want to say that there was no contradiction. When I quoted you, "the mixture is heated in my experiment. [...] I think longer
reaction time give higher yield," I suggested continuous heating. To rely solely in the heat of hydration (?) of the acid to develop the reaction was
one of the problems that I saw in the procedure.
What was the concentration of the acid you used? From the papers I've read (no patents, by the way), the acid hydrolysis with
H2SO4 is very sensitive to concentration, being fast when the concentration is above 80% and terribly slow when below 70%.
Considering that you added water to the acid, your final concentration was about 0.88 of the original strength, and after the addition of water it
could be too weak to completely hydrolise the PET in 1.5 hours.
The other question is, did you discard the solution? I'm still very interested in it because of the substances that may be there. One of them is BHET,
or bis(2-hydroxy-ethyl) terephthalate, which is one of the oligomers I described in February 6th. You can react BHET with a diacid, like phthalic acid
or even oxalic acid, to produce a polymer.
My suggestion is, use 20-25 mL of concentrated (83% or more) acid (no water added) for each 5 grams of PET with constant heating for 3-5 hours. With
concentrated nitric acid, you'll have oxalic acid rather than ethylene glycol, a more useful product. Neutralise the acid with NaOH, and the rest you
know better than I do.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@bnull: Yes I discarded the solution. No glycol recovery. Oxidise it to oxalic acid is a good idea.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
Heating is absolutely necessary. I tried this procedure with 20 g of finely chopped pet plastic and ~100 ml of sulfuric acid. The sulfuric acid was
hot from the water added, and most of the plastic dissolved into a clear, thick liquid. After letting it sit for a day, there was still some
undissolved solids so I decided to heat it. After a few hours at ~60 C, the solution turned white with precipitate. I think this is terephthalic acid
dropping out of solution due to the large amount of it. I then poured the slurry into about 500 ml of water and filtered out the terephthalic acid.
There looks to be a lot of it, definitely more than 5 grams. Currently it’s drying, I’ll report back after I dissolve in sodium hydroxide solution
and reprecipitate the terephthalic acid.
Also, while I was filtering it, there was a distinct smell of ethylene glycol.
[Edited on 2-20-2024 by Sir_Gawain]
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Ethylene glycol is odorless. Why not hydrolyzing PET directly in solution of NaOH and avoiding wasting H2SO4?
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
when distilling eg i have noticed a smell, unique and i can only describe it
has "leaking heater core" smell without the metallic tinge. But my source was antifreeze and likly to contain impurities
[Edited on 20-2-2024 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I am currently processing about 60g of coke bottles in concenrated NaOH solution (approx 4x excess). It has been sitting for over a week and has had
several hours of refluxing at about 110C during that time. A few chunks remain but it has mostly broken down.
I now have a white powder settled amongst the remaining fragments of unreacted PET. I am assuming this is the disodium salt which is undissolved due
to my liquid volume being so small and hence the solution concentrated. I am yet to do proper testing on the material. But another week or two
sitting in the beaker will not hurt it.
It is really nice to have an experiment that can just sit and do its thing while I have been caught up with work and family and other pressures.
No idea what I will do with the terephthalic acid once I have it. (The only use I know of is making PET and I don't think I need to do that.)
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by j_sum1  | | No idea what I will do with the terephthalic acid once I have it. (The only use I know of is making PET and I don't think I need to do that.)
|
Unfortunately it’s not a very interesting molecule. You can decarboxylate it to benzene, but that’s more
practical to do with sodium benzoate. You could convert it to the diacid chloride or the dimethyl ester, but these are just ways to turn a boring
compound into slightly less boring but still not too useful compounds.
|
|
|
| Pages:
1
2 |