dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
Tryptamine Acylation-Cylization
Please excuse my cursory ochem knowledge.
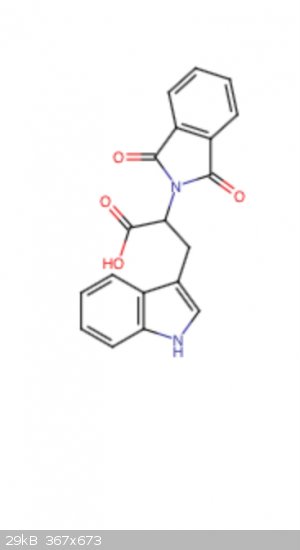
In a similar manner to the synthesis of 2-Aminoindan-1-one from phenylalanine, I thought that the preparation of the corresponding tricyclic
tryptamin-one could be a very interesting and easy experiment starting from tryptophan.
Basically, N protection of tryptophan with phthalic anhydride to the phthalimide, followed by PPA-promoted Friedel crafts acylation, and subsequent
hydrolysis of the phthalimide leaves the annulated tricyclic a-kept primary amine (tetrahydronorharmane but an annulated 2-aminocylopentanone instead
of piperidine).
I just don’t know if to the acylation would be preferential to the 2-position on indole (more resonance stable since aromatization wouldn’t be as
affected) or to the 4 position on indole. The 4 position ring closure would also induce a lot of torsional strain on the hypothetical product. 2 seems
to be more likely but I’m not well versed in this sort of thing so any help is greatly appreciated.
Thanks
[Edited on 22-2-2024 by dettoo456]
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
I made a mistake in the first post above; the ketone would actually be in the 3-position of the pyrrolidine ring. So you’d be left with the
1,2,3,4-tetrahydro-cyclopentaindol-2-amin-3-one.
It does seem like the acylation would only occur on the indole’s 2-position.
[Edited on 26-2-2024 by dettoo456]
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
| Quote: | | Basically, N protection of tryptophan with phthalic anhydride to the phthalimide, |
This may prove to be problematic, that is, using the most direct approach. In the Billman & Harding 1948 paper that was provided by Tsjerk in the thalidomide thread in Prepublication the preparation of the phthalyl derivatives of a large number of amino
acids is reported, all prepared by direct condensation with phthalic anhydride at 180°C for 15 minutes, in yields ranging from fair to excellent.
DL-phenylalanine is amongst them, giving the phthalyl derivative in 79% yield.
However, they mentioned that of all amino acids they tried, tryptophan, tyrosine, serine and taurine did not give the desired derivatives.
sic transit gloria mundi
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
I thought about that, but apparently it is possible in moderate yields if this paper (https://sci-hub.ru/https://link.springer.com/article/10.1007...) is to be believed.
And I figured in regards to my main question about the cyclization, that the indole’s 2 position would be greatly favored for acylation. I’m
primarily interested in the binding affinity of the final deoxygenated product - the rotationally constrained a-MT and its derivatives.
|
|
|