Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Michael addition of weak nucleophile
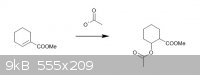
Hi,
Is it possible to do a michael addition of acetate to this molecule ?
thanks in advance
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not think anyone here can give you a definitive answer.
However, I can offer my humble opinion.
Nitroethylene can be condensed with trinitromethane (using methanol as a solvent). The two reagents are first mixed at 0degC, then refluxed to form
1,1,1,3-tetranitropropane. This reaction suggests that the reaction you propose should also work, with the carboxyl serving as the electron
withdrawing group across the unsaturated C=C bond. You would probably want to use acetic acid, not an acetate salt, even though most other Michael
addition reactions are conducted under alkaline conditions.
Also, nice user name! It reminds me of an email I got from someone who once worked under Olah. He was afraid I would kill myself working with methyl
perchlorate, not because it is liable to explode at slightest provocation, but because he thought it could likely be a potent alkylating agent,
similar in effect to the deadly "magic methyl".
[Edited on 4-5-2011 by AndersHoveland]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
not only is the nucleophile weak...but the Michael acceptor is weak as well. I don't think it will go.
Why not add HBr to the alkene and use it to alkylate KOAc? much better chance of working.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  |
Also, nice user name! It reminds me of an email I got from someone who once worked under Olah. He was afraid I would kill myself working with methyl
perchlorate, not because it is liable to explode at slightest provocation, but because he thought it could likely be a potent alkylating agent,
similar in effect to the deadly "magic methyl".
[Edited on 4-5-2011 by AndersHoveland] |
thanks !! A chemist told me he saw a black bottle with a huge skull drawn on it with the only one inscription "Methyl Magic", and the bottle will stay
here for years and years because no body dares touch it. I searched infos about that and I found a story about a dutch chemist who broke a 25 ml
bottle of MM in solution. He died after a few hours because the product made Methanol, H2SO4 and HF in his lungs... The product can also methylate
everything with a quantitative yield according to a old chemist I met.
| Quote: |
Why not add HBr to the alkene and use it to alkylate KOAc? much better chance of working.
|
SN2 on the cyclohexane moiety are very bad. I will probably go with the epoxide route,then acetic anhydride.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Actually, the high toxicity of "magic methyl" is due to the effect of CH3(+) ions (since fluorosulfonate is such a strong electron withdrawing agent).
Although the compound is covalent, it effectively acts as a donor of carbenium cations CH3(+), which alkylate bodily tissues. The effect is much more
potent than any of the HF that hydrolyzes off (and HF is decently toxic itself). I am not sure of the exact mechanism of toxicity, but I think it has
something to do with alkylation of DNA, which is very vulnerable to small base pair defects, resulting in cellular dysfunction and death.
This is one reagent that not every chemist should have access to (would not be surprised if someday chemists become required to obtain an
"extremely-hazardous reagent- handling" license before they are allowed to order these types of chemicals). Indeed, magic methyl has mostly been
phased out in favor of other safer reagents.
[Edited on 4-5-2011 by AndersHoveland]
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Hi, I found another way to make my molecule :

What is the cis/trans ration for the sandmeyer reaction ? I only need the cis product.
|
|
|