| Pages:
1
2
3
4
5
..
7 |
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
got somthing to help in the quest for purer sulphuric acid
See attachment:
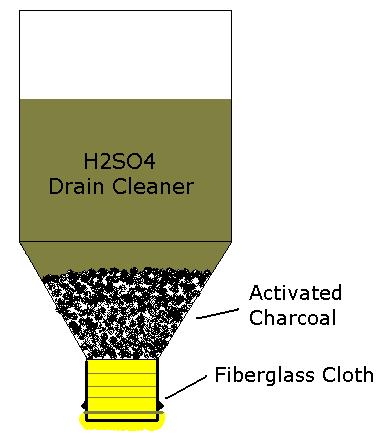
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Dont run on batteries. It'll cost you a fortune  If you have an old
computer that you're not using, take out its power supply, short the green to a black on the mobo connector and voila! Instant electrolysis
powersupply If you have an old
computer that you're not using, take out its power supply, short the green to a black on the mobo connector and voila! Instant electrolysis
powersupply  To run a cell, hook up a yellow or red for the positive and a
black for the negative. Check the key stuck on the power supply to see the voltages and amperages of each lead. To run a cell, hook up a yellow or red for the positive and a
black for the negative. Check the key stuck on the power supply to see the voltages and amperages of each lead.
Nice idea for the acid filter  But can we get activated charcoal here in drug
stores? Also, were could we buy fiberglass cloth But can we get activated charcoal here in drug
stores? Also, were could we buy fiberglass cloth 
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
I dunno, but i will check up real soon in the stores. As for your old computer idea, its good but will it damage the computer permenently? 
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Well, I heard that power supplys can get killed from overloading and stuff, but mine has been abused for a long time and its still alive 
Just take the power supply OUT of the computer to keep your hardware safe.
Ask axehandle, hes good with this stuff 
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Activated charcoal can be bought at aquarium suppliers. It comes quite economically and is capable of absorbing many ions, especially ones which cause
water to be coloured. What would its use be in filtering the H2SO4? To remove the colour?
And by the way, being prills, the charcoal can be place in a container with small holes at the bottom. No need for glass wool.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Sweet  I must check my local aquarium shop I must check my local aquarium shop 
Btw, how does it "absorb" ions? Does it react with the ions? 
Edit: About how many times can the carbon be reused?
[Edited on 31-3-2004 by Saerynide]
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
The absorption of activated charcoal works because 'acticvated charcoal' is highly porous and has a very large surface area to which it can
absorb chemicals. Activated charcoal absorbs mainly chloride ions and organic molecules. I don't think it reacts with the ions though, since it
is no form of ion exchange substance used in ion-exchange columns.
Activated charcoal is also sold in pet shops. I cannot find a specific quantity to be used. Just filter through the solution until all the colour has
been removed. When no change seems to occur replace the charcoal.
I am sure that certain ions like sodium and nitrates are not influenced by charcoal filtration, but ions like iron and chloride are. Activated
charcoal is used in gas mask to filter our certain chemical warfare agents (mostly organic ones) - Just to show you it absorbing capability.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Don´t wan to take the fun away Saerinyde but if you go to the forums ftp and download the "Kings Chemistry Survival Guide" you will find
under "electrochemical methods" exactly what you are after:
Electrolysis of MgSO4 to yield magnesium hydrate and dil. H2SO4.
The book is the hit anyways!
Highly recommended!
|
|
|
t_Pyro
Hazard to Others
  
Posts: 120
Registered: 7-2-2004
Location: India
Member Is Offline
Mood: Volatile
|
|
Silica gel is yet another good adsorbent. You can pass a solution through a column containing silica gel to adsorb the more easily adsorbed ions. To
"recharge" the silica gel, it has to be treated with dilute HCl to get rid of the adsorbed ions, then dried in a microwave oven.
Activated charcoal can be "recharged" by heating it gently, if I remember correctly.
Activated charcoal and silica gel "adsorb" substances by forming weak bonds (due to Van der Waal's forces) between the surface of the
adsorbent and the adsorbate. Finely divided metals adsorb certain gases by forming weak bonds with them at the surface.
Absorbents, on the other hand, "absorb", or incorporate into the bulk of the substance, the substance to be absorbed.
[Edited on 1-4-2004 by t_Pyro]
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
| Quote: |
Activated charcoal can be "recharged" by heating it gently, if I remember correctly.
|
No not gently, you need very high high temperatures. It must start glowing red hot for around 5 minutes.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
My god, thats like burning it. I might as well buy some new stuff cause it'll probably cost less than the gas needed to heat it 
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Ye you're right. Where I live aquarium fanatics used to 'reactivate' the charcoal by heating it in a baker's oven (the real big
ones) and said it worked, though they lose quite a lot of it. It isn't worth the trouble, better buy some when it is needed.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
I just read the part about electrolytic processes, and it said you have to "charge" the flower pot with MgSO4 even if you use electrolysing
NaCl or anything else. Whats the point of this? Wont it just lead to contamination?
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
I don´t think this will lead to contamination. Maybe the charging would also work with NaCl or something else, but I guess that the ions formed from
MgSO4 are most prone for permeating the pot and thats what the procedure is good for: Charging the pot with ions - once charged also other ions will
pass through with ease whats not sure - or may take a very long time otherwise.
Contamination is no issue regarding the amount of ions which will stay in the wall.....
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Progress!
I electrolyzed the contaminated H2SO4 for a few hours. The stainless steel knife was coated in a tan coloured stuff that looked like MgO which could
be easily scraped off the knife. Then I filtered the solution 4 times, using more and more filters each time. The acid is now almost clear, and the
pH has decreased slightly, from 4 to 3.5ish, judging from the indicator strip.
Tomorow, I will electrolyze it some more, then try to concentrate it to about half its volume 
|
|
|
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
I just had to give this a shot
I got a flower pot with the hole plugged and a plastic box where I placed 5000ml of cold tap water in the box and 1500ml in the pot. I took a sheet of
pglass and drilled a hole in the middle to support the electrode. About 330g of MgSO<sub>4</sub> was disolved into the water in the box
and 100g in the pot. both electrodes were made of lead from fishing weights.
I am running this at 5v/20amps. At first nothing seemed to happen but after a while small bubbles streamed off so the current densitry is low but this
seems to be ok. The pH of the outer solution is red at about 4 using my paper. The solution is beautifly clear no hint of contamination from the
electrodes. In the inner pot solution the pH is green at about 9 due to the pressance of Mg(OH)<sub>2</sub>. I don't see a
persipitate of Mg(OH)<sub>2</sub> yet but I have only run the cell for a few hours so it may be yet to come.
My cell is huge; 6500ml total! If I can get good cheap clear H<sub>2</sub>SO<sub>4</sub> from it then I may never buy the
stuff again! A good cheap source of Mg(OH)<sub>2</sub> will be handy as well. If this trial run is good then the "S Cell" may be
a permanent addition to the garage/lab area. I could just push it into the corner. 
The salt bridge Saerynide is using seems dubious to me. Filling it would be hard and it may contaminate the 2 solutions resulting in the formation of
more MgSO<sub>4</sub>!
Next day I will pipit off some liquid and do my "Acid Test"(pun intended) by heating my 200w soldering iron up and sticking the tip in the liquid. If all goes well I will see SO<sub>2</sub>
fumes(hopefully not so much as to injure me <b>again</b>! by heating my 200w soldering iron up and sticking the tip in the liquid. If all goes well I will see SO<sub>2</sub>
fumes(hopefully not so much as to injure me <b>again</b>!
Wait till the 'morro for the latest results!
What if, what is isn\'t true?
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Yeah, I mentioned earlier that the salt bridge might lead to an equilibrium being formed between the two cells.
| Quote: | | I just thought of something. There would be an equilibrium in the cell because the electrodes can only draw so many anions and cations to them at any
moment, so some ions would be crossing the salt bridge and reacting back with the acid and base to make the salt again, right? That would mean, the pH
can only go so low, and would level off. |
And my suggestion for solving it would be:
| Quote: | | So, if after each run, I filter the catholyte out to remove the Mg(OH)2, then run it again, and keep repeating this, I would eventually remove all the
Mg2+ from the cell? That would leave me with a salt free acid? |
The only I reason Im using this salt bridge is because I cant find an unglazed flower pot 
|
|
|
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
Living in Singapore can't be so good for free market capitalism; its the size of one of our states! It seems to me that rockwool inside pvc tube
would be a better bridge and that a wooden bowl could act as a better bridge but not for strong acids/bases! It might contaminate the solutions even
then. You might want to try a 'glazed' pot even then. I got one I thought was glazed(it felt smooth) but it works. Now if its shiny and a
difrent color then its a safe bet it realy is glazed
What if, what is isn\'t true?
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
I have lots of flower pots at home. There's an orchid farm near here and my mum likes gardening. Ok the pots are quite big with the ability to
hold at least 2L. I think i will get a small bucket to hold this. As for lead electrodes, its virtually impossible to get hold of them. So, copper
electrodes are ok? I can always recrystallise the CuSO4 produced and electrolyse them later.
Saerynide, you might want to try the orchid farms near the northern part of the island. They have a wonderful supply of pots and $10 each.
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
Incidentally, how much MgSO4 do i need to dissolve into the solution? Or will any amt do?
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
I'll give one of those orchid farms or those warehouse-sized flower shops a try. Probably wont have time to get one til around May cause of all
the exams coming up 
I heard that 30% salt (by wieght, I would assume) was best for electrolysis. I have no idea why though, maybe it has something to do with diffusion
and equilibrium? Maybe someone here can explain it to me or correct me 
|
|
|
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
Results!
I ran the cell over night and the pH is 10 for the Mg(OH)<sub>2</sub> and 2 for the
H<sub>2</sub>SO<sub>4</sub>! The pot has lots of milky
white Mg(OH)<sub>2</sub> coating the inside of it and on the bottom. After doing the Acid Test The pot has lots of milky
white Mg(OH)<sub>2</sub> coating the inside of it and on the bottom. After doing the Acid Test by sticking a hot soldering iron in it and looking for SO<sub>2</sub> I saw some! Now I am going to boil
the 5000ml down outside! by sticking a hot soldering iron in it and looking for SO<sub>2</sub> I saw some! Now I am going to boil
the 5000ml down outside!
Nearly free Sulfuric Acid at last!
What if, what is isn\'t true?
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
woooohoooo 
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
| Quote: | Originally posted by Quantum
I ran the cell over night and the pH is 10 for the Mg(OH)<sub>2</sub> and 2 for the
H<sub>2</sub>SO<sub>4</sub>! The pot has lots of milky
white Mg(OH)<sub>2</sub> coating the inside of it and on the bottom. After doing the Acid Test The pot has lots of milky
white Mg(OH)<sub>2</sub> coating the inside of it and on the bottom. After doing the Acid Test by sticking a hot soldering iron in it and looking for SO<sub>2</sub> I saw some! Now I am going to boil
the 5000ml down outside! by sticking a hot soldering iron in it and looking for SO<sub>2</sub> I saw some! Now I am going to boil
the 5000ml down outside!
Nearly free Sulfuric Acid at last! |
Good work! But you know how far you will have to boil this solution down? A pH of 2 implies 10^-2 moles of hydrogen ions per liter. At best, this
solution is 0.01 molar (and it is actually worse than that, because H2SO4 has two hydrogens to ionize). To get a 6 molar solution, which is the
concentration of battery acid, you will have to boil it down by a factor of 600! If you boil that down by a factor of 3 or 4 you will then have
concentrated H2SO4!
|
|
|
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
Damn!
I have not yet been able to boil it down but somehow I don't think my yeilds will be very good I may be better off with buying it but I called the only hydroponics store nearby and they don't know whats in
the acid solutions! They do however have 35%H<sub>2</sub>O<sub>2</sub>! I may be better off with buying it but I called the only hydroponics store nearby and they don't know whats in
the acid solutions! They do however have 35%H<sub>2</sub>O<sub>2</sub>!
Do you guys think its worth buying some "pH Down" and then testing it by heating a drop till I hopefully see SO<sub>2</sub>
fumes is a fairly safe bet? Im willing to try most anything.
What if, what is isn\'t true?
|
|
|
| Pages:
1
2
3
4
5
..
7 |