qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
NMR - Restricted Rotation
Hi Guys,
I was wondering why for the compound below do I see for the H-NMR that the two ethyl groups are not equivalent.
I know it is because of restricted rotation, but I am not too sure what is meant by restricted/hindered rotation.
I have attached a picture below.
Thanks!

|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Rotation cannot occur around the N=C bond.
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Ok, then why are the two ethyls attached to the nitrogen not chemically equivalent?
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
looks like resonance to me
Give me librium or give me meth!
Patrick Henry....
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Please explain further!
I don't understand why the two ethyls would be chemically different 
|
|
|
Dr.Bob
International Hazard
    
Posts: 2665
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
One ethyl is closer to a ketone, one is closer to an aromatic ring. The electronics of the neighboring groups will impact the magnetic field around
the ethyls, which is what the NMR is detecting.
The rotation around the N=C bond is slow enough to allow the two ethyl groups to each respond differently to the NMR. If they rotate fast enough
then the groups signal becomes the average of the two positions.
This is common for sterically hindered amides, biaryls and other molecules which are crowded around a C-C or similar bond.
Bob
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
I assume you mean magnetically equivalent?
Look up anisotropy. Aromatic systems create ring currents which generate their own electromagnetic fields. This is why the electrons attached to an
aromatic ring are shifted so far. Due to the resonance effects, the double bond of your amide system lies in the plane of the benzene ring and due to
resonance, rotation about the carbon-nitrogen bond is hindered. This means that one of the two ethyl groups will lie adjacent to and in the plane of
the benzene ring, exposing it to the deshielding effect of the ring current.
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Yes, sorry I meant magnetically equivalent.
Great explanation Dr.Bob, I enjoyed the "One ethyl is closer to a ketone, one is closer to an aromatic ring." Things can be so simple sometimes!
Without any rotation being allowed, there is a distinction between the two protons.
"The rotation around the N=C bond is slow enough to allow the two ethyl groups to each respond differently to the NMR." I guess I'd just have to see
the frequency of my NMR and see if my NMR machine is beast enough to be faster than the N=C rotation.
Thank you Fledarmus for adding more information.
This was great! Thanks! Crystal clear!
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
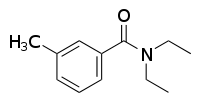
Would I be able to tell by looking at an NMR if the protons on the top ethyl would be more downfield or upfield than the protons on the bottom?
Thanks!
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
By looking? Only if you have a good grasp of the numerical values of anisotropic effects. You can imagine a shielding zone on both the aromatic ring
and the carbonyl double bond oriented into and out of the plane of the molecule - the methylene of the top ethyl is in the deshielded region of the
carbonyl, and the one on the bottom ethyl is in the deshielded region of the aromatic ring. Due to the presence of a ring current in the aromatic ring
system, my sense is that the effect on the bottom ethyl would be stronger, and those protons would be further downfield, but I am not in the business
of calculating NMR spectra and this is only a guess. In practice, I would run a NOESY and see which of the methylene signals resonated with the
aromatic proton signal.
|
|
|