qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Very Basic Carbonyl question
Why in the heck does NaOH deprotonate the alpha carbon and not act as a nucleophile for nucleophilic addition on the carbonyl carbon?
This is beyond my understanding.
Any explanation would be unbelievable.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Look into the acidity of protons near an electron withdrawing group. A bit less motivation to keep them there, yes?
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
Reactions are not all-or-nothing cases. Nucleophilic attack on carbonyl carbon in ketones happens, but is disfavoured by the steric hinderance or the
alkyl groups. If we would add a bulk base like LDA, would enolate formation be highly favoured. Formation of enolate is also kinetically favoured as
deprotonation is a really fast step. Overall the enolate product is stabilized by delocalization of electron density over the O=C-C bond. Based on
this we could say that enol formation is favoured over nucleophilic attack, at least when dealing with a non-activated compound.
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
Nucleophilic attack on carbonyl carbon in ketones happens, as kavu said, but the big question is what happens next? The resulting gem-dihydroxy
compound is unstable and eliminates water to reform the carbonyl. The dihydroxy compound is too unstable to be isolated (as is the enolate in most
cases) and there is no other reaction path available except the reverse reaction.
The enolate reaction is also reversible, but if there is something present that can react with a carbon anion, you give it an alternative reaction to
something that is more stable.
Both reactions occur, but the enolate reaction in most cases is the only one which can lead to a product different from the starting material.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
For highly halogenated aldehydes and ketones like trichloroethanal and hexafluoroacetone the equilibrium favours the diols which can be isolated.
http://en.wikipedia.org/wiki/Chloral_hydrate
http://en.wikipedia.org/wiki/Hexafluoroacetone
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fledarmus  |
Both reactions occur, but the enolate reaction in most cases is the only one which can lead to a product different from the starting material.
|
Ok, so if I were to take a ketone and react it with NaOH and I took the NMR/IR of the crude mixture would I be able to see that there would be a very
very small % of the gem-diol present?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
There are some exemplary cases where the "hydrate" is favoured over the carbonyl - sciencesquirrel touches on a couple (chloral is notably well known
for this) but other cases such as cyclopropanone exist in the hydrate form, due to reduction of ring strain (moving from ideal 120* at the carbonyl to
109.5* in the hydrate; wants to be 60* so any reduction is greatly favoured).
Note also that there are cases where the enol form of the carbonyl is favoured - I don't think you'll ever see this with an aldehyde, but in some
ketones (typically 1,3-dicarbonyls etc.) such as dimedone and acetoacetate esters there is a significant quantity of the tautomer.
If you take a ketone and treat it with a strong base such as NaOH, you'll likely get an aldol condensation occuring. With acetone, this means the
formation of mesityl oxide. A quick search using google will bear more detailed answers.
[Edited on 1-12-2011 by DJF90]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
For very acidic ketones like acetylacetone the presence of the enol form can be verified by spectroscopy.
Ooops, forgot the link!
http://en.wikipedia.org/wiki/Acetylacetone
You cannot detect the enol form or the gem diol of a simple ketone like acetone in aqueous solution as far as I know because they are present in very
small amounts.
[Edited on 1-12-2011 by ScienceSquirrel]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
You can show that reversible hydration occurs by using labelling studies with oxygen 18,
http://pubs.acs.org/doi/abs/10.1021/ja00961a013
and you can show keto - enol tautomerisation by deuterium exchange
http://www.youtube.com/watch?v=rKMBkmYdwes
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
This is awesome sciencesquirrel! Thanks!
I sent the same question to my professor... but it's been over 24 hours and he still hasn't answered me! But this makes a lot of sense now.
I sent this e-mail to my prof: Do you guys think I said many "stupid" things and that's why he didn't answer me?
Hi Dr.XXXXX,
I've been thinking about ketone reactions and I am slightly confused by the use of compounds such as NaOH/KOH and NaOMe in creating enolates from
ketones. I'll give you my understanding of what is going on, and I would highly appreciate it if you could correct me.
From my understanding, KOH and NaOH are strongly basic and nucleophilic. As has been engrained throughout this chapter, reacting KOH with a ketone
forms the corresponding enolate. However, KOH is also a nucleophile so it could also theoretically add to a ketone to form the corresponding gem-diol.
So my question is, why do we always seem to form the enolate instead of the gem-diol? I initially thought it had to do with equilibrium, but after
looking at the pka values of a ketone and water, the equilibrium lies to the left for both when KOH acts as a base to form the enolate or if it acts
as a nucleophile to form the diol! Why not just use LDA (strong non-nucleophilic base) to form enolates without having the problem of KOH or NaOH
reacting as a nucleophile?
I do agree that enolates can form alpha-beta unsaturated ketones which are stable, and like you said in class today, their formation drives the
equilibrium to the right. However, like you have mentioned all term, we don't have "smart" molecules so the KOH couldn't say to itself that it would
rather form the enolate than a diol because the enolate can then form the alpha-beta unsaturated ketone which is very stable due to
conjugation/resonance stabilization.
My only explanation of such a phenomena would be that both the gem-diol and enolate are being formed very quickly from the ketone. Knowing collisions
of molecules are random, an enolate might then collide with another ketone and then after a few more steps, a thermodynamically stable alpha-beta
unsaturated ketone would then be produced. This formation would then for all intensive purposes be almost irreversible because of the great stability
of the newly formed ketone and that this is the reason why KOH usually reacts with a ketone to form an enolate to a greater percentage than a
gem-diol. However, let’s say that I did the above-mentioned reaction in the lab (KOH reacting with a ketone) and took the crude mixture to the NMR
or IR machine; I'd be able to see that both an enolate and gem-diol (very small % of gem-diol) would be present in the crude mixture, right?
It's all about understanding the very basics so I hope that you can shutdown any of my wrong statements/assumptions. I tried googling my question but
I couldn't find anything. However, I did find that in the Cannizzaro reaction, OH is used a nucleophile in its first step but this was only because
there were no acidic hydrogens on the aldehyde.
Thanks!
XXXXX
Do you guys think I said many "stupid" things and that's why he didn't answer me?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Blush 
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Blush as in I should be embarrassed for asking that question to my professor?
Should I be embarrassed for making that statement to my professor? What do you guys think?! Was my question/statement really that stupid? Please
answer 
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I am blushing because of the praise.
Not even the girlfriend calls me awesome! 
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
Haha ScienceSquirrel! Too funny!
One other small question for you/the other guys on the forum. I have come to agree that NaOH will usually form the more stable enolate which can then
potentially aldol condense into a more thermodynamically stable product.
However, isn't there a possible reaction where the oxyanion of the hydrate then reacts with the ketone by attacking the electrophilic portion of my
ketone? I have attached a picture below.
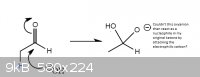
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You seem to be missing the salient point - hydration and enolisation both can and do occur, but both are equilibrium processes. Only the enolate can
react to form a product (aldol condensation) - the anion of the hydrate will just eliminate hydroxide to re-form the ketone. There is no process to
the best of my knowledge where a hydrate anion can be isolated or reacted to form a further product, except in two cases; the canizzaro reaction of
unenolisable aldehydes (dianion formed first), and in the final steps of the haloform reaction, in which case the hydrate anion kicks out CCl3(-)
instead of the usual OH(-) due to the better leaving group ability (lower pKaH).
|
|
|
qw098
Harmless

Posts: 22
Registered: 25-10-2011
Member Is Offline
Mood: No Mood
|
|
I understand it quite well DJF90, but I am trying to understand why! I understand that both reactions are occuring, and they are equilibrium
processes. But what prevents the anion of my hydrate to react with my original ketone?
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
What would happen if it did? You now have a di-hemiacetal-like system which again, doesn't have any productive reaction possibilities and unzips back
into the original starting materials.
I'm not sure you could state with certainty that this reaction doesn't occur, but I can't see any way of trapping the intermediate to prove that it
does. Maybe you could find it by running the reaction in the vapor state using flowing afterglow mass spec/mass spec to follow the generation of
transient intermediates...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by qw098  | Why in the heck does NaOH deprotonate the alpha carbon and not act as a nucleophile for nucleophilic addition on the carbonyl carbon?
This is beyond my understanding.
Any explanation would be unbelievable.
|
Your problem is in that you assume that something does not occur even though you have absolutelly no evidence. Why on earth do you think a hydroxide
does not add on the carbonyl groups? There are dozens of thousands literature examples of reactions that involve such an addition, yet you prefer to
ignore the literature and claim it does not happen. I'm puzzled at the ease of how you constructed such an assumption.
Quote: Originally posted by DJF90  | | There is no process to the best of my knowledge where a hydrate anion can be isolated or reacted to form a further product, except in two cases; the
canizzaro reaction of unenolisable aldehydes (dianion formed first), and in the final steps of the haloform reaction, in which case the hydrate anion
kicks out CCl3(-) instead of the usual OH(-) due to the better leaving group ability (lower pKaH). |
There are dozens of other reactions that also start with the addition of a hydroxide on the double bond of the carbonyl group in ketones or aldehydes.
There is the retro-aldol and retro-Claisen condensations, then there is the depolymerization of formaldehyde, the Grob fragmentation and surelly many
others that I can't bring to my memory at the moment. This is only for aldehydes/ketones, the nucleophilic addition reactions of the hydroxide anion
on other carbonyl groups are also possible and extremelly well known (the basic hydrolysis of esters, anhydrides, acid chlorides and amides are just
the best known ones). Like I said, I'm just don't understand what made qw098 believe that the hydroxide does not add on the carbonyl.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Sorry Nicodem, tunnel vision here...
|
|
|