Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Alternative routes, recycling of PET
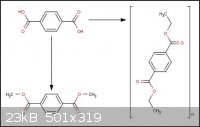
Recently i've been trying a new approach to the recycling of plastics, to be more specific poly(ethylene
terephthalate), by degrading it into its original constituents. So far that aspect has been successful and i have synthesized terephthalic acid and
ethylene glycol. The problem now is that i can't go about polycondensing these chemicals, in a suitable environment, to recreate the initial PET.
Additionally can someone give some guidance as to the preparation of alkyl terephthalate esters like methyl and ethyl terephthalate.
Can someone offer some assistance please, i have a deadline by which to complete my research experiment.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
ive used the plastic and melting it with a plastic welder harbour fright the plastic welder uses a stream of heated air it can melt PVC PVP
Polycarbonate at around 850F the hot air can burn paper
or at least blacken it melts a PET bottle easily adjusting the hot air flow will keep it from burning ive casted some things in plastic.
there are other things ive heared with plastic ive heared of backyard chemistry turning it into oil
http://www.youtube.com/watch?v=qGGabrorRS8
another http://www.youtube.com/watch?v=BbKSSr9ypts&feature=relat...
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Plastic Recycling
The two videos are quite inventive and will help in my secondary fuel reconstitution research. However, i need
to stick to the polycondensation of the mentioned compounds as that is the current primary objective.
Thanks for the post, i got some new ideas to experiment on for the fuel research project though.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I suspect the best approach to breaking up PET for recycling is via high pressure methanolysis.
http://www.jproeng.com/qikan/manage/wenzhang/2011-017.pdf
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
What sorts of problems are you having? Both the preparation of methyl and ethyl terephthalate and the preparation of PET are simple esterification
reactions. Methyl and ethyl esters are typically prepared by dissolving the carboxylic acid in methanol or ethanol and adding a catalytic amount of a
strong mineral acid (usually H2SO4). Methanol usually works very well, for ethanol you may also have to add something to suck up the water that is
given off.
Polymerization is a somewhat different story, and there is an enormous patent and non-patent body of literature devoted to the best ways to prepare
polyesters.
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Information requisition
The problem is that the two constituents don't seem to be polymerizing during the experiment. I need a way to
create the ideal environment to induce polymerization and polycondensation of the 'monomers'.
Can you please specify where the literature on polyester formation can be located? The request for help on the formation of the terephthalate esters
was so that i could get a guidline which to follow in the experiment, other than what i already know. Things such as specific experiment temperature
and synthesis precautions would be helpful, thanks for the reply.
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
Well, the original patent gives several different preparative examples - US Pat 2465319. A search in Google patents gives about 1500 hits on
polyterephthalate.
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Thank you
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
thats why they use ethylene oxide and terephthalic acid 
|
|
|
Chemistry Alchemist
Hazard to Others
  
Posts: 403
Registered: 2-8-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Polyethylene Terephthalate combined with Sodium hydroxide and heated until all the plastic dissolves, will produce Ethylene glycol and Sodium
Terephthalate which then can be added to HCl to produce Terephthalic Acid and Sodium Chloride... small scale doesn't really work will for this, larger
scale would be better (100grams of PET would be good, i only done 10 grams so my yield of terephthalic acid was low)
|
|
|
Ionic Chemist
Harmless

Posts: 39
Registered: 22-3-2011
Member Is Offline
Mood: Polymerizing
|
|
Thanks to everyone for the help
@Chemistry Alchemist
Yeah that is the usual method that I use to synthesize terephthalic acid its pretty simple to carry out and I have access to a lot of PET and sodium
hydroxide.
@Fledarmus Thank you for the information its really good. Now I am going to synthesize polymers; consisting of amide substituted carbonyl groups
(O=C-NH2)connected to nitrated aromatic rings (C6H5-NO2), with various polyols.
Thanks for the replies.
|
|
|