Dope Amine
Harmless

Posts: 39
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
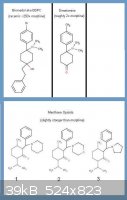
Opioid Aminobenzycyclanols derived from Menthone
I'm hoping that there might be somebody here who is familiar with the SAR for unconventional opioids such as: bromadol (BDPC), dimetamine, ciramadol,
tilidin, or tramadol. This knowledge then might be transferable to the proposed compounds I have drawn in the picture below. These are based off of
similar compounds derived from menthone and cited in the article: Aminobenzylcylcanol Opioids
It is explained in the pictures, but basically my questions are:
1. Why not make the dimethylamine material instead of one of the cyclic amines? Methadone (dimethyl) is twice the strength of the similarly
structured dipipanone (piperidine) and other compounds related to the title compounds are all dimethylamines (tilidine, tramadol, ciramadol).
2. In the article, the ketones are stronger than when converted to alcohols, but what if instead we attached a phenethyl group at the same location
that we convert to the alcohol (via Phenethyl-Li or Phenethyl-Mg-Cl)? Might that prove more potent as is the case with the same reaction on
dimetamine?
3. How about putting a para-methyl or Br on that benzene ring like bromadol/dimetamine?
This article in pretty cool just from the standpoint that the title compounds, opioids which are slightly stronger than morphine, are fairly quickly
made from benzaldehyde + menthone (w/NaH), and then your choice of cyclic amine. Short n' sweet.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2660
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
One problem is that I have found that Phenethyl halides tend to eliminate in basic conditions to form styrenes which then polymerize to tar. I was
trying to do chemistry like that years ago for a different series and got yields in the 5% range of the product, and still had about 7 steps to go to
get to the desired product, which turned out to be a poor anti-inflammatory agent, much as I had predicted. So unless you want to convert pounds of
starting material into milligrams of product, this might not be so good. And of course benzyl grignards are also sometimes bad actors, as they can
reorganize into orthotolyl materials in some cases.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
i wonder if that aldol condensation could be acid catalyzed it seems like that would also work.
Give me librium or give me meth!
Patrick Henry....
|
|
|
Dope Amine
Harmless

Posts: 39
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
Phenethyl halides: I got a very good yield reacting phenethyl-Br with 4-piperidinol to get N-phenethyl-4-piperidinol, so I'm not familiar with the
issue you speak of. The only bummer with that route was that then I had to do a damn Swern oxidation to get to the desired N-phenethyl-4-piperidone
because my previous chromium oxidation caused the organic molecule to complex as a ligand. This really is a whole different subject though. For
attaching a phenethyl group at the ketone carbon, I would recommend using phenethyl-Li instead of phenethyl-Mg-Br or the like. Not sure why you
mentioned benzyl Grignards...
As for the aldol condensation, I dunno if acid catalysis would be viable. They certainly needed a very strong base to go at it that route.
Thanks for the thoughts...
[Edited on 8-1-2012 by Dope Amine]
[Edited on 8-1-2012 by Dope Amine]
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
next time try MnO2.
or you could make the ethylene ketal of piperidone and n-alkylate that.
yeah i'm pretty sure acid catalysis would work.
reason why is i don't like the idea of usong sodium hydride and ether.
one drop of water gets in there and you're in trouble.
[Edited on 9-1-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
There just looking for a strong base if you are scared of NaH then NaNH2 or better yet Sodium Nitride should do the trick just as well if not better.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
oh i'm sure there are plenty of ways to do that.
you know it occured to me you could forego the oxidation to the piperidone if you made the anilino piperidine from the tosyl or mesyl ester of the
piperidinol and aniline, yeilds would be good too, probably quantitative.
[Edited on 9-1-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|