safety first
Harmless

Posts: 5
Registered: 4-1-2012
Member Is Offline
Mood: No Mood
|
|
cheap source of quartz tubes?
Hi everyone, I've been lurking around reading and learning but didn't have anything to say until now. So my question is have you seen these neat
little quartz tubes used in cheap radiant heaters? They have a small diameter, maybe 8 to 10mm but they could probably be used for some high temp
syntheses. And manufacturers claim them to be fused quartz. Anyone ever used one? I'm interested in making lots of high temp things but don't have a
backyard or barbecue to play with so these seemed like a good idea.
Any thoughts?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Yes, that seems to be fused quartz, however for some reason it isn't transparent, but kind of opalescent.
I heated it using a propane-butane torch and it remained unaffected. I've never tried an actual synthesis using them, though.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Free source of quartz tubes?
I've seen toaster ovens that appear to have <a href="http://en.wikipedia.org/wiki/Fused_quartz" target="_blank">fused quartz</a> <img
src="../scipics/_wiki.png" /> tubes surrounding the metallic heating elements.
<strong>More than you ever wanted to learn about commercial lighting:</strong>
At my job, I often replace <a href="http://en.wikipedia.org/wiki/High-intensity_discharge_lamp" target="_blank">HID lamps</a> <img
src="../scipics/_wiki.png" /> in <a href="http://www.informinc.org/fact_P3high_bay.php#what" target="_blank">high bay</a> <img
src="../scipics/_ext.png" /> fixtures. Most of these <a href="http://en.wikipedia.org/wiki/Lamp_(electrical_component)"
target="_blank">lamps</a> <img src="../scipics/_wiki.png" /> (the correct term for a <a href="http://www.techlinea.com/wp/?p=181"
target="_blank">'light bulb'</a> <img src="../scipics/_ext.png" /> have an inner fused quartz envelope called an arc tube (see <strong>Fig. I</strong>
have an inner fused quartz envelope called an arc tube (see <strong>Fig. I</strong> . These contain most of the harmful metals and halogens inside an unexploded HID lamp. Approximately half the lamps (the ones needing
replacement) that I deal with have had the arc tube explode (on a good day, the outer glass envelope remains intact). These partially exploded lamps
are collected in designated cardboard drums and are destined for recycling by the lighting supplier (same with <a
href="http://en.wikipedia.org/wiki/Fluorescent_lamp" target="_blank">fluorescents</a> <img src="../scipics/_wiki.png" /> . These contain most of the harmful metals and halogens inside an unexploded HID lamp. Approximately half the lamps (the ones needing
replacement) that I deal with have had the arc tube explode (on a good day, the outer glass envelope remains intact). These partially exploded lamps
are collected in designated cardboard drums and are destined for recycling by the lighting supplier (same with <a
href="http://en.wikipedia.org/wiki/Fluorescent_lamp" target="_blank">fluorescents</a> <img src="../scipics/_wiki.png" /> . .
If they've completely exploded, I chuck 'em in the dumpster outside—since most of the Hg and other 'nasties' have already vaporized into
the atmosphere inside the building. I prefer to keep my occupational Hg vapor exposure as low as is feasible. So, my thoughts are why concentrate
the vapor inside the barrel or building when it's already destined for release into the environment?
<center><table><tr><td><!--<img src="http://amerilights.com/uploads/misc/pg57_image_hid.jpg" width="300" alt="Figure I"
caption="Figure I" />--><img src="../scipics/user:bfesser/HID-Lamp.gif" alt="Figure I" caption="Figure I"
/></td></tr><tr><td><strong>Figure I.</strong> Diagram of a typical HID
lamp.</td></tr></table></center>
Some of the smaller HID lamps that I deal with in track lighting contain an extra glass component. It's an extra cylindrical tube surrounding the
inner envelope (see <strong>Fig. II</strong> . I'm not sure, but I
think this tube may also be constructed of fused quartz. When these lamps (with the extra tube) cease to function properly, the arc tube often
remains intact. When I replace them, I often wonder if it would be worth it to cut off the outer glass envelope in order to recover the cylindrical
glass tube surrounding the arc tube. These tubes are likely too short for most laboratory applications, but does anyone here know if this component
is indeed fused quartz? . I'm not sure, but I
think this tube may also be constructed of fused quartz. When these lamps (with the extra tube) cease to function properly, the arc tube often
remains intact. When I replace them, I often wonder if it would be worth it to cut off the outer glass envelope in order to recover the cylindrical
glass tube surrounding the arc tube. These tubes are likely too short for most laboratory applications, but does anyone here know if this component
is indeed fused quartz?
<center><table><tr><td><img src="http://www.vosslighting.com/storefrontB2BWEB/img/staging/HID/mh_ps_ed28p_cl.gif"
width="300" alt="Figure II" caption="Figure II" /></td></tr><tr><td><strong>Figure II.</strong> Photograph of
a Philips MP175/BU/12PK.
Note the cylindrical glass tube wrapped in a wire helix.</td></tr></table></center>
<strong>Final personal note:</strong>
I'm very happy that my employer has finally begun to phase out all of the <a href="http://en.wikipedia.org/wiki/Halogen_lamp#Handling_precautions"
target="_blank">halogen</a> <img src="../scipics/_wiki.png" />, <a href="http://en.wikipedia.org/wiki/Compact_fluorescent_lamp"
target="_blank">compact fluorescent</a> <img src="../scipics/_wiki.png" />, and <a
href="http://en.wikipedia.org/wiki/Incandescent_light_bulb" target="_blank">incandescent lamps</a> <img src="../scipics/_wiki.png" />.
The new <a href="http://en.wikipedia.org/wiki/LED_lamp" target="_blank">LED lamps</a> <img src="../scipics/_wiki.png" /> are
fantastic; longevity, energy consumption, ease of recycling, etc. As an added bonus, I receive less nasty burns and cuts at my day job. Those
injuries are now reserved for chemistry. 
[edit: the first image has been replaced since this was originally posted, because the original image was hosted externally and disappeared]
[Edited on 7/9/13 by bfesser]
|
|
|
Morgan
International Hazard
    
Posts: 1661
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
http://www.technicalglass.com/product_pages/fused_quartz_lab...
http://www.technicalglass.com/product_pages/fused_quartz_tub...
http://www.advaluetech.com/clear_fused_quartz_tubing.html
http://www.quartz.com/quartz.html
http://www.fdglass.com/store?filter_tag=17
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Bfesser - I've read contradicting articles (wikipedia included) about the efficiency of LED vrs Halogen and HID lighting
Which is more efficient?!
It seems like the "Oldies" like to say LED's are a hoax, while the "newies" say that halogens are old news.
ʃ Ψ*Ψ
Keepin' it real.
Check out my new collaborated site: MNMLimpact.com
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
These radiant heating rods are fused quartz that has not been completely degassed, thus the translucent appearance. It is equal to the more expensive
transparent fused quartz regarding physical and chemical properties, the appearance is the only difference.
I'm sure they could come in useful for chemical reactions, but their small diameter limits the batch size a lot.
You can bend them and melt them shut with an oxy-propane welding torch (wear dark glasses!).
I've made a small batch of phosphorus (less than 1g) from phosphate, silica and aluminum in a quartz tube (OD ca. 12mm, melted shut at one end and
plugged with kaowool on the other end) once, by heating it with a welding torch on the outside. This way, the required temperature of 1500°C can be
reached with ease, and a good yield is obtained.
The downside is that the quartz tube is ruined after one batch since the phosphate/silicate slag fuses to the quartz and the different coefficient of
expansion shatters the tube upon reheating. Since I used a good, expensive piece of transparent quartz this experiment was not repeated.
If you have access to old space heaters or toasters with quartz tubes for free, maybe you could try this experiment.
bfesser, the cylindrical shrouds around the arc tubes in HID lamps are indeed fine transparent quartz. No other glass would stand up to the
temperatures so close to the arc tube.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by garage chemist  | | bfesser, the cylindrical shrouds around the arc tubes in HID lamps are indeed fine transparent quartz. No other glass would stand up to the
temperatures so close to the arc tube. |
garage chemist, I don't mean to offend, but do you have a source to support this claim? I'd just like to be sure before I put any more effort into
this.
If that is indeed the case, I have a free source of as many of these tubes as I can recover. Of course, I would leave the arc tube intact and send it
in for proper recycling/disposal. I'm thinking that the best way would be to cut the outer envelope cleanly (at a point where the diameter is just
large enough to pull the quartz cylinder out) using the method employed by the gentleman in <a
href="http://www.youtube.com/watch?feature=player_detailpage&v=gl-QMuUQhVM#t=263s" target="_blank">this familiar video</a> <img
src="../scipics/_ext.png" />.
Procedure outlined:<ol><li>Cut outer envelope at red line.</li><li>Pull entire assembly out of 'bulb'.</li><li>Cut
wires as necessary to remove quartz cylinder (highlighted in blue).</li><li>Salvage any useful <a
href="http://en.wikipedia.org/wiki/Tungsten" target="_blank">W</a> <img src="../scipics/_wiki.png" /> wire.</li><li>Recycle
the arc-tube and <a href="http://en.wikipedia.org/wiki/Getter" target="_blank">getter</a> <img src="../scipics/_wiki.png"
/>.</li></ol>
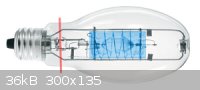
I wonder how difficult it would be to fuse these end to end. Should I give it a try?
[hehe, it looks like I had a stutter]
[Edited on 7/9/13 by bfesser]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Read this:
http://www.budgetlighting.com/shat-r-shield/pdfs/arctube.pdf
I originally had the information about the HID lamps with additional quartz shroud from the german Osram site (these are called HQI-E with the E
standing for explosion-protected) but there were no english sources on the Osram site, or I didn't see them.
Anyway, if anyone wants quartz tubes and reaction vessels for serious experiments like SO3 production then I can only recommend going to a technical
glassblower with a drawing of what you need, and have it custom made.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
If you can do a butt
joint already in borosilicate glass, then yes. If not, practice with borosilicate. You'll almost certainly need a glass lathe to get a straight joint.
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
All these halogen lamp bulbs also are made of quartz, and they are clear as a bonus. http://static.traderscity.com/board/userpix47/18152-118mm-Li...
|
|
|
safety first
Harmless

Posts: 5
Registered: 4-1-2012
Member Is Offline
Mood: No Mood
|
|
Thank you guys for the input, I'm sure it will prove valuable. I think I'm going to give one of these tubes a shot for an 'easy' pyrolysis, meaning a
not really toxic one, although I haven't really decided in one yet. 
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Magpie has done it! Conversion of an HID lap for use as an intense UV source by removing the envelope, here: <strong><a
href="viewthread.php?tid=14927#pid306354">How to make Carbon Tetrachloride</a></strong>
|
|
|