Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
glyme
Anticipating a future need for glyme ( dimethyl ether ethylene glycol, DME) I began a search for same. I could not find it for sale from any of my
usual sources nor could I find any consumer goods from which it could be extracted in useful quantities.
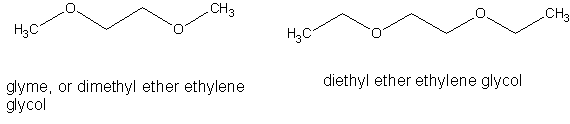
Looking at the structures of glyme and its ethyl analog it can be seen that they are both diethers. I'm wondering if these could be synthesized from
ethylene glycol using a Williamson ether synthesis. Methyl iodide and ethyl bromide seem like possible alkylating agents.
According to Wiki this is not the standard route to glyme. So, what do you think? Would this work? What would be the likely difficulties?
[Edited on 29-3-2012 by Magpie]
[Edited on 29-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Methyl iodide and ethyl bromide seem like possible alkylating agents. |
If you have them handy, seems like it would be relatively easy to make an attempt. But both of those are so volatile that it seems like you'd want to
do it under containment... speaking for myself I might try to do it the other way around, using sodium methoxide or ethoxide and 1,2-dichloroethane.
Would allow for temps 30 degrees higher.
The less you bet, the more you lose when you win.
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
I don't know if this will be of any use, but dichloroethane can be obtained cheaply <a
href="http://www.elementalscientific.net/store/scripts/prodView.asp?idproduct=1847">here.</a> This may make synthesis of glyme more
economical.
Sodium ethoxide thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=2656
[Edited on 29-3-2012 by barley81]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bbartlog  | | speaking for myself I might try to do it the other way around, using sodium methoxide or ethoxide and 1,2-dichloroethane. Would allow for temps 30
degrees higher. |
I could find nothing of this sort in the literature. Not even with 1,2-dibromoethane. Instinctively, I would say the product of such a reaction would
be vinyl chloride. I did a rapid literature search and indeed there are references confirming that heating 1,2-dichloroethane in alcoholic hydroxides
or alkoxides does give vinyl chloride, in some cases nearly quantitatively (I only read the abstracts, but they were all very convincing). One of the
first (or perhaps the very first) syntheses of vinyl chloride was done this way (Justus Liebigs Annalen der Chemie, 1935, 14, 30). The same
happens to 1,2-dibromoethane under such conditions (Organic Letters 2011 13, 2090).
Perhaps it would be possible to react 1,2-dibromoethane with methanol under solvolytic conditions using NaHCO3 as a base at reflux. This should
prevent the elimination, but the substitution would most likely take an eternity (non-deprotonated methanol is a lousy nucleophile).
Otherwise, there are several references for the dimethylation of ethylene glycol in the literature: with MeI in Na/NH3 (Organic Letters 2010,
12, 5186); with (MeO)2SO2 in Na/EtOH (J. Chem. Soc. 1912, 101, 1804); with MeI in Na/EtOH (Justus Liebigs Annalen der Chemie 1983,
276, 182).
The most convenient syntheses are acid catalyzed etherifications. There is plenty of literature, mostly patent applications, on these methods, but
unfortunately I don't have the time to review hundreds of references. Sulfuric acid can also be used though it is not particularly efficient (DE3446488). It gives a mixture of mostly methyl cellosolve, glyme and dioxane, but ethylene glycol is cheap and methyl celosollve is also useful
(if nothing else it can be reworked in the same reaction to give a better yield of glyme).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Hmm. I know I've seen K2CO3 plus dichloroethane used somewhere (adding to some molecule resembling catechol, the reference for which I can't find
now), but you're right: that was for an aryl compound, where I guess it works because the aryl compound is a million times more acidic and therefore
readily deprotonated by K2CO3 (where methanol/ethanol would not be). My bad.
The less you bet, the more you lose when you win.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | I could find nothing of this sort in the literature. Not even with 1,2-dibromoethane.
...
Perhaps it would be possible to react 1,2-dibromoethane with methanol under solvolytic conditions using NaHCO3 as a base at reflux. This should
prevent the elimination, but the substitution would most likely take an eternity (non-deprotonated methanol is a lousy nucleophile).
|
I found one example: US3699174! It wasn't abstracted in the reaction index of SciFinder and Beilstein, so I missed it earlier. It is the reaction of
1,2-dichloroethane with methanol under solvolytic conditions with ZnO, MgO, CaO or other as the basic buffers for HCl scavenging. The problem of the
reaction rate is addressed in the usual way - by increasing the reaction temperature to 200 °C (note that the syntheses based on ethylene glycol
etherifications are usually also done at similar temperatures). Best yields are obtained using the least basic buffer, zinc oxide. The major side
product is 2-methoxyethyl chloride.
The same reaction should be applicable to 1,2-dibromoethane at considerably lower temperatures. I would estimate that 140-160 °C could be enough.
With iodide based nucleophilic catalysis, the required reaction temperature might be even lower.
|
|
|