mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Chlorination of an aromatic ring
What would be some viable methods of chlorinating this compound:
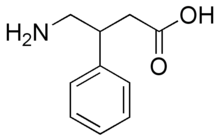
at the para position on the benzene ring? I assume the amino group will form an intermediate with the Cl atom and consequently, the compound would get
chlorinated at the ortho position. I suppose that could be prevented by protecting the amine with a bulky protecting group. Any other problems one
might encounter in their attempt to chlorinate only at the para position? Maybe if bulky protecting groups are attached to the amine and the acid
group then these protecting groups would sterically block the ortho and meta positions, leaving only the para position open.
[Edited on 3-4-2012 by mycotheologist]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
So you're trying to prepare a substituted β-(p-chlorophenyl)ethylamine? What purpose do you have for the target compound?
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
I'm learning about EAS reactions and decided to pick that GABA analogue as a precursor for a thought experiment. The fact it has an amino group as
well as a carboxyl group poses a challenge.
[Edited on 4-4-2012 by mycotheologist]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mycotheologist  | | I assume the amino group will form an intermediate with the Cl atom and consequently, the compound would get chlorinated at the ortho position. I
suppose that could be prevented by protecting the amine with a bulky protecting group. |
The chaperon effect in chlorination is only observed in nonpolar solvents and freebase amines. Neither of this conditions is possible due to the
nature of your substrate and its solubility issues.
| Quote: | | Any other problems one might encounter in their attempt to chlorinate only at the para position? Maybe if bulky protecting groups are attached to the
amine and the acid group then these protecting groups would sterically block the ortho and meta positions, leaving only the para position open.
|
A bulky group on the amine is already too distant to have a worthy enough steric influence on the ortho/para selectivity. Your substrate already has a
relatively bulky aromatic substituent so there is not much else to do here. It is however poorly activated for an electrophilic aromatic substitution
and chlorinations are poorly ortho/para selective. The chlorination of the related cumene commonly gives a 1 : 1 ratio of the ortho vs. para
chlorinated product, so I would expect a small excess of the para isomer in your case. Chlorination in water might give a better para selectivity due
to the charge and consequent strong solvation around the substituent, but this is just guesswork.
In regard to any need for the protecting groups, the carboxy group is not really a problem and the amino group can be protected by protonation. In
principle, you might get a good monochlorination selectivity by chlorination in aqueous sulfuric acid. You could try with either chlorine or
trichloroisocyanuric acid (TCCA) as the electrophilic chlorinating reagents (chlorine, being a gas, is not as easy to quantify, though). I would try
with 20-60% H2SO4(aq) as a solvent and TCCA in about 20% excess.
There is no easy way to monitor such a reaction, except if you have an HPLC method suitable for such amino acids. There would be precipitation of
cyanuric acid if you use TCCA in dilute H2SO4, but this does not necessarily correlate with the desired reaction pathway. There are some types of TLC
plates that could work, but the more common silica plates are not suitable as far as I know. The isolation would be difficult as you would have to
catch the isoelectric point. The separation of the desired para-chloro product would also be difficult if the reaction would not have enough para
selectivity to allow a separation via recrystallization (in any case the yield would suffer from the isolation losses).
PS: Please open referenceless threads only in the Beginnings section. Once you equip the thread with a minimum of references (if you UTFSE you can use
plenty already on this forum) I might get interested enough to complement with further literature data.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
4-4-2012 at 08:43 |