| Pages:
1
2 |
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
"in my eyes the reaction youve drawn will stop at the imine", if so then the eschweiler-clark mechinism is wrong, the R group cannot be a hydrogen or
it wont work due to the other functional groups on the molecule, or other random shizzle, methyl lithium is of no practical cancer risk, it will
explode in air before having chance to form damaging hydronium ions in your cells
[Edited on 29-5-2012 by rannyfash]
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rannyfash  | "in my eyes the reaction youve drawn will stop at the imine", if so then the eschweiler-clark mechinism is wrong, the R group cannot be a hydrogen or
it wont work due to the other functional groups on the molecule, or other random shizzle, methyl lithium is of no practical cancer risk, it will
explode in air before having chance to form damaging hydronium ions in your cells
[Edited on 29-5-2012 by rannyfash] |
okay mehtyllithium isnt carcinogenic but how do you prepare LiMe? =)
and oops i forgot you mentioned the Eschweiler-Clarke mechanism... After i had a look into i think its worth a shot.
it doesnt really matter where the imin comes from... so if the conditions are right it should react like youve drawn! =)
but only the experiment will proof this! as i said before im looking forward to it!
best regards
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
methyl lithium?, i prepared a subgram quantity, around 0.7g (too dangerous and scared to make larger amounts), methyl bromide from(ish) http://www.youtube.com/watch?v=7Jn0z5BGpR0 baring in mind bromomethane boils just above 0 celcious, dissolved in diethyl ether and reacted slowly
with lithium in an air locked (air lock filled with mineral oil) container with very little air space, replaced air lock with rubber septum very fast
and carefully, a fire starts as soon as the waterlock is removed, i think its the most dangerous compound ive ever made, considerable amount was lost
to trace amounts of water, and then a glass syringe can be used to transport it safeishly, i did no tests to decipher if it really was methyl lithium
the fact it created a flamethrower out the end of a syringe was good enough for me  , i took precautions , i took precautions
[Edited on 30-5-2012 by rannyfash]
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rannyfash  | methyl lithium?, i prepared a subgram quantity, around 0.7g (too dangerous and scared to make larger amounts), methyl bromide from(ish) http://www.youtube.com/watch?v=7Jn0z5BGpR0 baring in mind bromomethane boils just above 0 celcious, dissolved in diethyl ether and reacted slowly
with lithium in an air locked (air lock filled with mineral oil) container with very little air space, replaced air lock with rubber septum very fast
and carefully, a fire starts as soon as the waterlock is removed, i think its the most dangerous compound ive ever made, considerable amount was lost
to trace amounts of water, and then a glass syringe can be used to transport it safeishly, i did no tests to decipher if it really was methyl lithium
the fact it created a flamethrower out the end of a syringe was good enough for me  , i took precautions , i took precautions
[Edited on 30-5-2012 by rannyfash] |
lolz well done but methylbromid is carcinogenic you know. =)
after some more reading i also think the eschweiler-clarke wont work because the mechanism uses an iminium-ion and not an imine.
best regards
[Edited on 30-5-2012 by Organicus]
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
this is on wikipedia, i hope it is correct, the imine ion is initiated by the formic acid
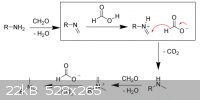
[Edited on 30-5-2012 by rannyfash]
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rannyfash  | this is on wikipedia, i hope it is correct, the imine ion is initiated by the formic acid
[Edited on 30-5-2012 by rannyfash] |
have a look at this:


what do you think?
best regards
[Edited on 30-5-2012 by Organicus]
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
hmmmm, maybe a trace of strong acid for a catalyst then? assuming formic acid is not strong enough to protonate the imine, as long as the imine is
protonated the reaction can still occur, i had a quick browse over google images and couldnt see any other major variations
|
|
|
Organicus
Harmless

Posts: 15
Registered: 29-5-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rannyfash  | | hmmmm, maybe a trace of strong acid for a catalyst then? assuming formic acid is not strong enough to protonate the imine, as long as the imine is
protonated the reaction can still occur, i had a quick browse over google images and couldnt see any other major variations |
youre right and formic acid should be strong enough to protonate the imine. for protonating imines usually a mix of AcOH/AcNa is used. my concerns are
ruled out now, so lets have a look into the praxis. =)
best regards
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I think this is closest to the original questioner's intentions, note the use of protecting groups.
http://www.erowid.org/archive/rhodium/chemistry/amphetamine....
|
|
|
rannyfash
Hazard to Others
  
Posts: 113
Registered: 21-2-2012
Member Is Offline
Mood: No Mood
|
|
its a shame phosgene is too dangerous for me to work with
|
|
|
| Pages:
1
2 |