Vlassis
Harmless

Posts: 9
Registered: 16-10-2011
Location: Athens
Member Is Offline
Mood: Ok.
|
|
Sulfonation Of Phenol
The other day I was reading an article concerning the synthesis of catechol by reacting molten potassium hydroxide with
orthophenolsulfonic acid.I was wondering if the reaction is even possible, since the difference between melting points of those two compounds
is around 250 degrees.I could not find the exact reaction somewhere, so I thought it might be this:
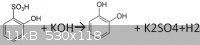
I forgot a 2 in front of the potassium hydroxide
[Edited on 22-5-2012 by Vlassis]
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Arylsulfonic acids react with molten alkali by forming alkali phenoxides and sulfites. Hydrogen gas is not produced. Typically, an alkali sulfonate
salt is used instead of the sulfonic acid.
ArSO<sub>3</sub>K + 2 KOH -> ArOK + K<sub>2</sub>SO<sub>3</sub> + H<sub>2</sub>O
Hypothetical reaction of the dipotassium salt of o-phenolsulfonic acid and KOH equation:
C<sub>6</sub>H<sub>4</sub>SO<sub>4</sub>K<sub>2</sub> + 2KOH ->
C<sub>6</sub>H<sub>4</sub>(OK)<sub>2</sub> + K<sub>2</sub>SO<sub>3</sub> +
H<sub>2</sub>O
Check Vogel's Practical Organic Chemistry in the SM library for more information on arylsulfonic acids and fusion of their salts with alkali. I'm not
sure if the compound you mention is a special case.
[Edited on 22-5-2012 by barley81]
|
|
|
Vlassis
Harmless

Posts: 9
Registered: 16-10-2011
Location: Athens
Member Is Offline
Mood: Ok.
|
|
Thank you for your reply.I will check out Voguel, but do you know any possible synthesis of the ortho and not the para sulfonic acid.I know that you
must keep the temperature low, but how low exactly?And after that, how to seperate the ortho para and meta sulfonic acids formed?
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
From Thorpes Dictionary of Applied Chemistry:
Pyrocatechol may be obtained in a pure condition by fusing one mole of phenol ortho sulphonic acid with twenty four moles
of caustic potash at 320 to 330 degrees Celsius. The yield is twenty percent of the theoretical.

Chemistry is our Covalent Bond
|
|
|
Nicodem
|
Thread Moved
24-5-2012 at 11:04 |