Electrolysis of dilute HCL using Pb electrodes.
Inspired to try this from a few ideas on the Pb salts prep thread, but posted in here as interesting observations , and maybe nice way from preparing
some of the more insoluble salts , from the more unreactive metals.
[img]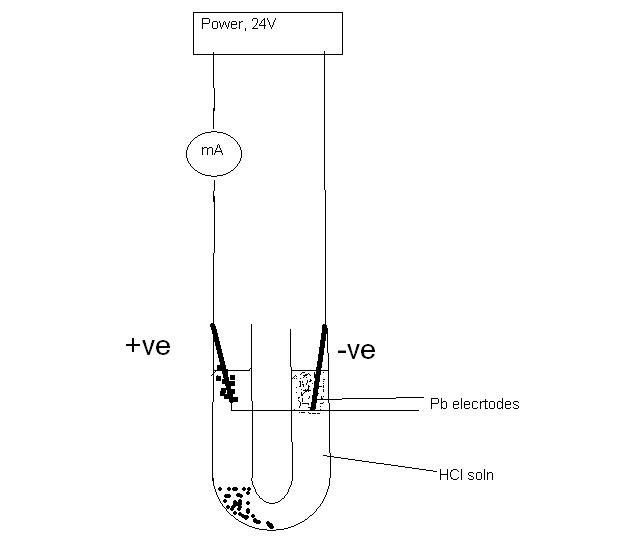 [/img] [/img]
( SORRY NO CAMERA! ) )
Observations:
1) the Pb anode had PbCl2 forming fairly fast at first, snap, crackes and pops were heard, ( PB2+ ion reacting directly with Cl- ions.......violent
reaction????? )
2) the solution at the anode side got very hot after 5 / 10 minutes( measured up to 80'C, and the increased solubility of the PBCL2 caused less of
the crystals to be seen. After cooling ,small silky white needles of PbCl2 were seen to form on wall of U tube and on anode.
3) very little, if no Cl2 gas was produced at the anode.
4) the current fell as the PBCL2 retarded the contact of current flow from the anode to the solution. ( remained at about 300mA,) . Current mostly
stable at about 450 mA mosty of time)
Current rapidly fell from about 2000mA when experiment first started.
Upon gently scraping the PbCl2 from the anode, more pops , crackles were heard, and the needle, (or the digital readout on multimeter) twiched up in
unison with the crackles.
5) the crackles at anode were favorable, as this seemed to permit the higher current, and faster PbCl2 production, these crackles were enough to allow
dislodging of more PbCl2 and sinking down vessel.
6) the cathode bubbled freely and spongy Pb accumulated. The temperature at the cathode rose MUCH less than at the anode.
I would say that the reaction of the Pb2+ ions formed at anode must have been pretty exothermic, with direct reaction with the Cl- ions, and the temp
rose at anode accordingly., If the solution getting pretty hot, was due to the heating current effect of current, then the whole U-tube would have
been the same-ish temp.
I have repeated exp in a 100 ml beaker within in a 400ml beaker with cold water for a cooling jacket, More PbCl2 produced, due to colder solution,
but needs recrystallization ,as small bits of spongy lead have contaminated it slightly,
Nice effect watching the snow flakes of heavy PbCl2 appear from nowhere and flutter their way down the limb of the U-tube.
|