| Pages:
1
2 |
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
1,3,5-trichlorohexahydrotriazine
I made my first energetic compound!
I do not really like energetic compound but following a post of franklyn I found an energetic compound that I wanted to try.
http://www.sciencemadness.org/talk/viewthread.php?tid=2945&page=3
The process was not well explained, but the general idea was that if hexamine is reacted with NaOCl/CH3COOH mix it will make
1,3,5-trichlorohexahydrotriazine ( C3H6N3Cl3).
Experimental:
(I did all the process outside)
2.5g of hexamine from coleman fuel tablet was disolved in 25ml of water. Then 30ml of 10% sodium hypochlorite (pool chem) was mixed with 40ml of 10%
acetic acid (cleaning vinegar). After pouring the NaOCl/CH3COOH mix in the hexamine sol. the solution turned cloudy white, I swirled and suddenly a
yellow flocculent formed on top. I filtered it and take a pinch of it and try to dissolve in water but iit was insolube in water, so water was a
perfect solvent to clean the product. I cleaned the product with water and I lets it dry. After drying I deflagrated some of it, It burn instantly
making a pop sound, success!
Maybe I will try to make a salt with this compound.
Description of the product (from my own analyse):
Yellow material insoluble in water and smelling chloramine.
Thanks!
I never asked for this.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Sorry for the double post.
This compound seam to be very energetic when dry. The product take some time to dry but when it is completly dry it is quite impressing for someone
that never made energetic compound. The compound is solube in methanol.
The most impressive with this compound is the OTC(ness) of its starting material.
edit:
I'm precipitating a bromo variant. More info on the process/reactivity soon.
[Edited on 2-6-2012 by plante1999]
I never asked for this.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
If you are willing to experiment , I'm wondering if RDX can be achieved by this
method. NaNO2 sodium nitrite is also very soluble in methanol. Mixed with the
compound you have made and promoted with some lewis acid catalyst such as
CuCl2 copper chloride , it could result that NaCl would precipitate out producing RDX.
. .NCl --- CH2
. . / . . . . . . . \
CH2 . . . . . NCl . . . . . + 3 NaNO2 => 3 NaCl + C3H6N6O6
. . \ . . . . . . . /
. .NCl --- CH2
Those of you with more knowledge of synthesis please contibute your opinion
and critique this. It's been a nagging thought for too long.
Hypothetically NaClO3 sodium chlorate is also worth trying , possibly yielding a
Perchloramide >NClO3
.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I'm willing to experiment but I do not have sodium nitrite. I have sodium chlorate.
The product generate chlorine when in 30%HCl probably this reaction is appening:
6HCl + C3H6N3Cl3 + 3H2O -) 3Cl2 + 3NH4Cl + 3CH2O
I never asked for this.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Just exactly how many explosive options hexamine gives you  ? I'll
remember-hexamine-NaClO-acetic acid for when I have NaClO ? I'll
remember-hexamine-NaClO-acetic acid for when I have NaClO
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by Ral123  | Just exactly how many explosive options hexamine gives you  ? I'll
remember-hexamine-NaClO-acetic acid for when I have NaClO ? I'll
remember-hexamine-NaClO-acetic acid for when I have NaClO |
NaOCl can by bougth at the hardware store as ''liquid chlorine''. If you cannot get some look for 65% calcium hypochlorite or '' shock chlorine'' for
pool it should also work.
[Edited on 3-6-2012 by plante1999]
I never asked for this.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I can't afford basic lab equipment as scale and use mostly jars... At the price of the NaClO I'd buy 2kg of good AN...
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | I'm wondering if RDX can be achieved by this
method.
Those of you with more knowledge of synthesis please contibute your opinion
and critique this. It's been a nagging thought for too long.
|
That is an intiguing question. One thing for certain, however, it would not work if there was any water present. The chloramine would act like a
bleach and the nitrite would get oxidized to nitrate.
I am not entirely sure how the N-Cl bond would behave in nucleophilic substitution reactions. Although the N-Cl bond can readily hydrolyse in water,
it could be much less reactive in an attempted substitution reaction with nitrite.
I believe this may be anologous to the reactions of haloalkanes with sodium nitrite. Chloroalkanes generally do not react, while bromoalkanes are much
more reactive. Remember, the proper solvent needs to be used, which is both non-nucleophilic (generally water is not optimal) and can dissolve the
sodium nitrite. DMSO is a good solvent for this. If trichlorotriazine is unreactive, you might try tribromotriazine.
You might see this reference:
http://www.sciencedirect.com/science/article/pii/S1044030500...
It mentions that nucleophilic substitution on chloramine to form chloride ions proceeds at a rate more than 100 times that of chloromethane, which may
still be rather slow. Consider for example that the hydrolysis of chloromethane with water takes around a whole year!
| Quote: |
Anhydrous chloramine is an extremely hazardous (toxic and explosive) and unstable (decomposes at −50 °C) compound. Therefore, it was prepared
in situ in an aqueous solution according to the modified Raschig method: Aqueous ammonia (30%) and a solution of sodium hypochlorite (11%–13%
available chlorine), both chilled to −18 °C, were mixed in a 1:4 ratio by volume (a slight excess of hypochlorite) in an ice/NaCl bath. The
resulting fresh solution of chloramine (10 mL, ≈3 M; kept in an ice/water bath at 0 °C) was immediately used for experiments
|
[Edited on 4-6-2012 by AndersHoveland]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Like I said earlier I'm willing to experiment but i do not have NaNO2 or DMSO solvent. I could buy some...
At least I could make anhydrous aluminium bromide as a catalyst.
I also tried to make bromo variant but it did not seam to work... I will make hypobromite soon and attempt to make it again.
[Edited on 4-6-2012 by plante1999]
I never asked for this.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Not sure if the below relates to this topic:
| Quote: |
The nitration of aliphatic secondary amides and of primary and secondary aliphatic amines has been studied extensively and some understanding of the
reaction is now at hand. Firstly it has been found that the ease of nitration in acetic anhydride varies inversely in respect of increasing base
strength. Secondly those strong amines which cannot be nitrated directly can be converted to their nitramines via the intermediate preparation of the
chloramines, presumably because the chlorine substituent reduces the base strength of the amine. In many cases the chloramine may be prepared in situ
in the acetic anhydride - nitric acid medium by adding a small amount of hydrogen chloride. The hypochlorous acid thus produced forms the chloramine
and is regenerated when the chloramine is converted to the nitramine. A chain reaction is thus developed which is largely self-sustaining. Finally
primary amines, which cannot be nitrated directly, may be converted to primary
nitramines via the primary dichloramine. The alkylchloronitramine (which can be converted separately to the alkylnitramine) is the actual product of
the latter reaction. This is fortunate because primary nitramines decompose in nitration media, often violently, in strong sulphuric or nitric acid.
In acetic anhydride or acetic acid the decomposition rate is dependent on the amount of excess nitric acid which is present.
MECHANISM OF GUANIDINE NITRATION
George F. Wright
Canadian Journal of Chemistry, Volume 30
|
Could someone look into this excert?
| Quote: |
The method was later worked out by Brian and Lamberton [38] to produce previously unobtainable nitramines. The formation of nitramines through
chloramines
Urbanski
|
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I tried this little experiment as well, but I obtained a white flocculent solid, on top of the liquid. As Plante1999 said, the liquid first becomes
opaque and white, and after swirling a flocculent solid is formed which moves to the top of the liquid. This solid, however, is white in my case,
white like snow.
@Plante1999: What kind of vinegar did you use? Was it clear colorless, or some colored and spicey stuff? In my experiment I used diluted acetic acid
from a chemical supplier and I used plain household bleach for the hypochlorite. I used purified hexamine, recrystallized from so-called ESBIT solid
fuel tablets. The hexamine I used has a fishy smell. Maybe the yellow color you observed is due to some impurity?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by woelen  | I tried this little experiment as well, but I obtained a white flocculent solid, on top of the liquid. As Plante1999 said, the liquid first becomes
opaque and white, and after swirling a flocculent solid is formed which moves to the top of the liquid. This solid, however, is white in my case,
white like snow.
@Plante1999: What kind of vinegar did you use? Was it clear colorless, or some colored and spicey stuff? In my experiment I used diluted acetic acid
from a chemical supplier and I used plain household bleach for the hypochlorite. I used purified hexamine, recrystallized from so-called ESBIT solid
fuel tablets. The hexamine I used has a fishy smell. Maybe the yellow color you observed is due to some impurity? |
My hexamine was from Coleman fuel tablet, 12.5% sodium hypochlorite and 10% house hold vinegar. But I think you are right since washing the product
with excess water give my a white floculent solid and a yellow solution. I think the impurity is chlorinated organic since they are quite volatile,
the yellow solution turn colorless in a few hour after the reaction.
I never asked for this.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I think it would be much preferable not to use vinegar. While vinegar and bleach certainly can form some hypochlorous acid in solution, the acetate
(from the vinegar) may be vulnerable to oxidation.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The vinegar is essential. Without the vinegar, no precipitate is formed at all. I tried that as well. Any weak acid will do the job, as long as it is
not oxidized by the bleach. Acetic acid is suitable, it resists oxidation very well.
|
|
|
niertap
Hazard to Self
 
Posts: 76
Registered: 5-8-2011
Member Is Offline
Mood: hyper-conjucated
|
|
[Edited on 9-9-2012 by niertap]
Ignorance is bliss
Outliers in life are modeled by chemical kinetics
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Well I just scored some NaNO2.
I am going to try making some trichlorohexahydrotriazine.
Plante1999 and Woelen .... Did you find any significant gas evolution or fuming besides the smell of bleach ?
Reason I ask is .... I reside in a fairly populated area with houses and lots of neighbors.
Smell of bleach .... No one cares about.
I am going to use 5% vinegar and hypo and double the amounts.
Thanks
|
|
|
Sydenhams chorea
Harmless

Posts: 29
Registered: 16-8-2009
Member Is Offline
Mood: No Mood
|
|
I am somewhat disappointed. It so happens I was at the uni library digging up papers on organic reactions with hexamine. My first thoughts were how
closely it resembled trichlorotriazine (cyanuric chloride), a powerful chlorination reagent which can substitute for thionyl chloride and PCl3 and has
the additional advantage of being a solid.
As above mentioned compound, trichlorohexahydrotriazine, is very closely related I was hoping to find similar capabilities for ths product.
Il n'y a point de sots si incommodes que ceux qui ont de l'esprit.
François de La Rochefoucauld.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by Motherload  | Well I just scored some NaNO2.
I am going to try making some trichlorohexahydrotriazine.
Plante1999 and Woelen .... Did you find any significant gas evolution or fuming besides the smell of bleach ?
Reason I ask is .... I reside in a fairly populated area with houses and lots of neighbors.
Smell of bleach .... No one cares about.
I am going to use 5% vinegar and hypo and double the amounts.
Thanks |
This process gives off quite some nasty smell. It is manageable, but I would not like to do it in a crowded area. There is a very peculiar smell, not
like bleach, but much more pungent. It probably is due to formation of some chloro amines. The hexamine I used has a fishy smell (it always has, due
to formation of trace amounts of methyl amines) and I think that the chlorination of these leads to the nasty and choking smell. You could try it on a
testtube scale first (e.g. 1/10 of the amounts, used by plante1999).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by AndersHoveland  | Not sure if the below relates to this topic:
| Quote: |
The nitration of aliphatic secondary amides and of primary and secondary aliphatic amines has been studied extensively and some understanding of the
reaction is now at hand. Firstly it has been found that the ease of nitration in acetic anhydride varies inversely in respect of increasing base
strength. Secondly those strong amines which cannot be nitrated directly can be converted to their nitramines via the intermediate preparation of the
chloramines, presumably because the chlorine substituent reduces the base strength of the amine. In many cases the chloramine may be prepared in situ
in the acetic anhydride - nitric acid medium by adding a small amount of hydrogen chloride. The hypochlorous acid thus produced forms the chloramine
and is regenerated when the chloramine is converted to the nitramine. A chain reaction is thus developed which is largely self-sustaining. Finally
primary amines, which cannot be nitrated directly, may be converted to primary
nitramines via the primary dichloramine. The alkylchloronitramine (which can be converted separately to the alkylnitramine) is the actual product of
the latter reaction. This is fortunate because primary nitramines decompose in nitration media, often violently, in strong sulphuric or nitric acid.
In acetic anhydride or acetic acid the decomposition rate is dependent on the amount of excess nitric acid which is present.
MECHANISM OF GUANIDINE NITRATION
George F. Wright
Canadian Journal of Chemistry, Volume 30
|
Could someone look into this excert?
| Quote: |
The method was later worked out by Brian and Lamberton [38] to produce previously unobtainable nitramines. The formation of nitramines through
chloramines
Urbanski
|
|
This is off for AH regarding the George F. Wright article in the Canadian Journal of Chemistry here is the article pdf
http://www.nrcresearchpress.com/doi/pdf/10.1139/v52-008
and here is some information on the precursor azobisformamidine
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Woelen .... Thank you for the heads up.
I'll try just reacting a quarter tablet or something.
Will let you guys know what happens...
I have this weird gut feeling that only 1 or 2 out of 3 N-Cl will convert to N-NO2 by treatment with NaNO2 in MeOH.
Just seems too easy. Only one way to find out if it will work or not.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
http://www.researchgate.net/publication/27256427_Synthesis_o...
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
I just reproduced the reaction with bleach and white vinigar using pure hexamine from a pyrotechnics suppliers. worked like a charm and produces a
snow white product. drying now. A very fun and easy reaction. It was easy to manage, but i did notice a very pungent smell as woleon describs. Thanks
guys.
[Edited on 26-9-2012 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
The related monomer
Chloroamine Reactions : Methylene Chloroamine
has also text on 1,3,5-trichlorohexahydrotriazine.pdf
www.sciencemadness.org/talk/viewthread.php?tid=9319&page...
You need to have access to the References section.
Yield is poorer due to perishability of chloramine precursor reagent.

From the SciMad Library Formaldehyde J. Frederic Walker page 123
When ammonium chloride and formaldehyde are added to hypochlorites.
the methylene derivative of monochloramine (CH2:NCl) is obtained in the
form of needle-like crystals.[ 21 ]
[ 21 ] J. Chem. Soc., 97, 2404-2406 (1910); Chem. Zentr., 1911, I, 236.
Cross, C. F . , Bevan, £ . J., and Bacon, W.
From the SciMad Library Formaldehyde J. Frederic Walker page 290
Reactions with Halogens and Inorganic Halides. Chlorine decomposes
hexamethylenetetramine in aqueous solution with formation of the explosive
nitrogen trichloride[ 29 ] With sodium hypochlorite, chloro derivatives are
obtained. These products are apparently unstable and may explode on storage.
According to Delepine[ 30 ], N-dichloropentamethylenetetramine, C5H10N4Cl2,
may be obtained in the form of thin leaflets by addition of a dilute sodium
hypochlorite solution to a solution of hexamethylenetetramine.
On heating to 78-82ºC, the product explodes. Leuher[ 29 ] reports a
dichlorohexamethylenetetramine (m.p. 77ºC) produced by addition of 8.4g
potassium bicarbonate and 45 cc sodium hypochlorite (72 g active chlorine
per liter) to 5 g hexamethylenetetramine in 40 cc water, claiming a yield of
77-80 per cent. A moist sample of this product prepared in the writer's
laboratory exploded violently on standing. A tetrachloro- derivative,
C6H8N4.Cl4 is claimed by Buratti[ 13 ].
[ 13 ] Buratti, R., Swiss Patent 90,703 (1921); Chem. Zentr., 1922, IV, 891.
[ 29 ] Delepine, M., Bull. soc. chim. France (3), 17,390.
[ 30 ] Delepine, M., Bull. soc. chim. France (4), 9,1025 (1911).
____________________________________________________
Substitution of the chlorine has been tried before
Some old bones _
www.sciencemadness.org/talk/viewthread.php?tid=9370#pid10889...
www.sciencemadness.org/talk/viewthread.php?tid=9370#pid10904...
www.sciencemadness.org/talk/viewthread.php?tid=9370#pid10906...
www.sciencemadness.org/talk/viewthread.php?tid=9370#pid10906...
related reference
www.sciencemadness.org/talk/viewthread.php?tid=4282&page...
If this reaction scheme can be made to work it opens
new areas of investigation for high nitrogen content
compounds such as terazole _
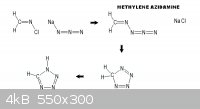
_ other ways to get it.
www.organic-chemistry.org/synthesis/heterocycles/tetrazoles....
http://heterocyclist.com/2012/02/13/tetrazole-synthesis-part...
http://heterocyclist.com/2012/02/15/tetrazole-synthesis-part...
________________________________________________
This is off topic , but while on the subject here is another
way to Tetrazole by way of methylenediamine precursor.
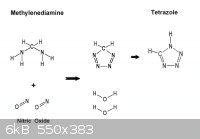
From the SciMad Library Formaldehyde J. Frederic Walker page 207
the preparation of methylenediamine salts* by the action of an excess of strong acid
on methylenediformamide at low temperatures. When 77 grams methylenediformamide
were added to 500 grams concentrated hydrochloric acid at approximately 12°C,
[ Knudsen 64 ] obtained 57 grams methylenediamine hydrochloride, CH2(NH2)2-2HCl
as a crystalline precipitate. Formic acid is obtained as a by-product of this reaction:
* Methylenediamine is of particular interest since it is the ammono- analog of
methylene glycol. Knudsen found that salts of this diamine are stable in the dry state
and, in addition to the hydrochloride, prepared the nitrate [ CH2(NH2)2.2HNO3 ]
and the sulfate [ CH2(NH2).H2S04 ] by reacting methylenediformamide with an excess
of concentrated nitric acid and 50 per cent sulfuric acid, respectively, at low temperatures.
The nitrate of methylenediamine crystallizes in prisms, which melt and then explode
when heated on platinum foil. Attempts to prepare methylenediamine itself by the action
of alkali on the hydrochloride were unsuccessful, since the pure base is apparently
unstable in the free state. However, when it is liberated in the presence of alcohol,
solutions of methylenediamine are obtained and, according to Knudsen, are sufficiently
stable for use in chemical reactions.
[ 64 ] Knudsen, P., Ber., 47, 2698-2701 (1914).
.
[Edited on 27-9-2012 by franklyn]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
If 1,3,5-trichlorohexahydrotriazine is energetic, it makes me wonder why 1,3,5-trichloro-1,3,5-triazine (TCCA) cannot be detonated.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Probably the oxygen atoms instead of hydrogen can stabilise the molecule.
Rest In Pieces!
|
|
|
| Pages:
1
2 |