ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Nucleophilic Substitution
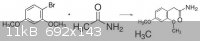
I was thinking would it be possible to do an enolate subtitution(nucleophilic substitution) of ethanamide with 1-Bromo-2,4-dimethoxybenzene. If the
following reaction is successful, then a clemmensen reduction will get rid of the ketone. The result would be 2,4-dimethoxyphenethylamine.
"Imagination is more important than knowledge" ~Einstein
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
CH3 is not a good leaving group ever... Just sayin
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
Br- is the leaving group..that methyl must be a typo.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Oh I see it now. You would need a suitable catalyst to remove that Br
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ChemistryGhost  | | I was thinking would it be possible to do an enolate subtitution(nucleophilic substitution) of ethanamide with 1-Bromo-2,4-dimethoxybenzene.
|
The pKa of primary amide N-H is many magnitudes lower than that of its alpha C-H, so it is not possible to obtain an enolate by a monodeprotonation of
acetamide.
Otherwise alpha arylations of tertiary amide derived enolates are known. See the literature. Off course, they are not simple nucleophilic
substitutions, only formaly so. These reactions proceed by either via photochemical nucleophilic substitution (rarely preparatively useful, unless
intramolecular) or via oxidative insertion, ligand exchange and reductive elimination (and thus require a transition metal catalyst). For this later
type of arylations, see the chapter Direct Arylation via Cleavage of Activated and Unactivated C-H Bonds (M. Miura, M. Nomura) published in
Topics in Current Chemistry 2002, 219 .
| Quote: | | then a clemmensen reduction will get rid of the ketone. The result would be 2,4-dimethoxyphenethylamine. |
There is no ketone carbonyl group in the putative product that you depicted. I believe you don't know what a ketone is. Please read about ketones.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Quote: Originally posted by Nicodem  |
The pKa of primary amide N-H is many magnitudes lower than that of its alpha C-H, so it is not possible to obtain an enolate by a monodeprotonation of
acetamide.
|
What if we protected the NH2? Would the single leftover hydrogen still have a lower pKa then the methyl's? I'm trying to think back to my
Organic 2 class
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
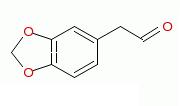
1-chloro-3,4-methylenedioxybenzene to 3,4-methylenedioxy-phenylacetaldehyde
Can 1-chloro-3,4-methylenedioxybenzene react with acetaldehyde in an enolate substitution reaction to form 3,4-methylenedioxy-phenylacetaldehyde. It
seems likely. 
3,4-methylenedioxy-phenylacetaldehyde.
"Imagination is more important than knowledge" ~Einstein
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Go read up on aromatic reactivity and mechanisms and you'll quickly see that this is impossible.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Nicodem
|
Threads Merged
26-10-2012 at 23:07 |