morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
4-bromobutanoic acid from 5-bromo-1-pentene
My book tells me I can use hot KMnO4, H2O, and NaOH to cleave the double bond, oxidizing to make CO2 and 4-bromobutanoic acid + MnO2 + H20 + ?
From here will it be okay to add NaOH again to cause an SN2 reaction at the primary halide, leaving a primary alcohol, or will I need to switch from a
protic to an aprotic solvent?
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
There are several ways to get the title compound, also KMnO4 could work, but not as described, hot KMnO4 will end up with a lot CO2 and non of what
you need.
I would suggest 3 route, 1 for because it's an awesome reaction and a few other because it is effective:
1: ozonolysis, make the ozonide with some ozone and work it up with az oxidizing agent.
2: KMnO4, OsO4, H2O2, tBuOOH and a lot other reagents are widely used to make 1,2 diols from unsaturated carbon chains with mild conditions, here I
would say to make the diol (1-bromo-pent-4,5-diol) and crack the chain over there with some sodium-periodate or Pb(IV)-acetate, ect.
A recipe for the formation of the diol:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0217
3: Make a water addition to the double bond with dilute H2SO4 in water, get the alcohol and oxidize it to the corresponding carboxylic acid with HNO3,
cat. NH4VO3. The only problem with this that the chain can also crack at the 3-4 carbon, so it's not a selective method.
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv1p0018
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
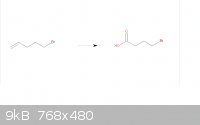
I considered your first option, it's on the same page in my book as the KMnO4 technique I brought up. If I were to pursue this option, should I use
KMnO4 immediately after treatment with Zn and acetic acid?
I'm aiming for a one-pot reaction here.
[Edited on 31-12-2012 by morry-cule]
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by morry-cule  | If I were to pursue this option, should I use KMnO4 immediately after treatment with Zn and acetic acid?
I'm aiming for a one-pot reaction here. |
Accoring to my experience one-pot reactions looks nice on papaer, but they usually end up with ablack gunk...
And could you describe why would be the Zn and the acetic acid used? What is needed to be reduced over there?
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
The Zn and acetic are replacements for the Me2S needed in the reaction. That's what the book says, and Zn/acetic acid is easier to get than Me2S. The
book I speak of is Organic Chemistry 10th edition, Wiley.
So I guess perhaps I should just ask you how you yourself would go about getting 4-bromobutanoic acid. Where would you start (not with
5-bromo-1-pentene?), etc.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by morry-cule  | The Zn and acetic are replacements for the Me2S needed in the reaction.
So I guess perhaps I should just ask you how you yourself would go about getting 4-bromobutanoic acid. Where would you start (not with
5-bromo-1-pentene?), etc. |
Ahhh, I see, reductive workup for the ozonation. That will work.
If I would have to make that halogenated carboxylic acid I would start from gamma-butyrolactone and a simple treatment with HBr would give the title
compound.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
Gamma-butyrolactone in my experience is hard to come by. I've made it from GABA before, and I've seen it offered overseas, but I understand it to be
quite watched.
|
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
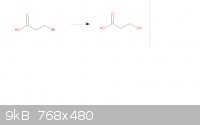
3-Bromopropanoic Acid Substitution
Turning 3-Bromopropanoic acid into 3-Hydroxypropanoic acid.
3-Bromopropanoic acid + NaOH + (acetone???) ----> 3-hydroxypropanoic acid + NaBr + (acetone???)
So for the solvent, I was thinking either acetone or ethyl acetate. Which one would work best, or is there another obtainable solvent that would be
better? Any help is much appreciated.
[Edited on 6-1-2013 by morry-cule]
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
Why not use aqueous NaOH? It should do the trick. There is also the possibility of elimination side product...
[Edited on 6-1-2013 by kavu]
|
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
Well where am I gonna find H20 at this time of night!?? ;D
Thanks, and as long as I keep the temperature low enough, E2 should be minimized. (says my book) The reason I didn't opt for H20 was because the book
says to use a polar aprotic solvent. Ah well. Aqueous solution it is!
Also I wrote a note in my book about a year ago that SN2 reactions using alkyl Cl/Br can be catalyzed with NaI or KI, would that be worth the effort
to add one of those?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
First of all, H20 is not a compound. The empirical formula for water is H2O. If you don't know that, you certainly should not be playing with
chemicals.
| Quote: | | Thanks, and as long as I keep the temperature low enough, E2 should be minimized. (says my book) |
Your book is full of bullshit. Such intramolecular nucleophilic substitutions that form five-membered rings don't give elimination products. The
kinetics for the cyclisation are way faster than for the elimination, especially where the nucleophile is such a weak base like the carboxylate in
this specific case.
| Quote: | | The reason I didn't opt for H20 was because the book says to use a polar aprotic solvent. Ah well. Aqueous solution it is! |
This is a science forum, so start using references!
And don't crosspost on the same topic. I'll merge it with the old one anyway.
| Quote: | | Also I wrote a note in my book about a year ago that SN2 reactions using alkyl Cl/Br can be catalyzed with NaI or KI, would that be worth the effort
to add one of those? |
I don't believe "the book" says that about the cyclisation of 3-bromopropionic acid to gamma-butyrolactone and its subsequent hydrolysis. What
reference to primary literature it gives anyway?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Threads Merged
7-1-2013 at 08:03 |
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
Hey Nicodem, that response of mine pertaining H20 was a JOKE - making light hearted conversation, hence the ;D at the end and the fact that I was
asking for a source of WATER, kinda like in an episode of the Simpsons where they take expeditions to get ice because the crew can't think of a
better way to make it. Jeez...
I don't think the book I'm using (Organic Chemistry 10th Ed, Wiley) is bullshit, it's probably the fact that I didn't realize it would always cyclize
like that. This is in chapter 6, and the only problems they give that show SN2 cyclization are with 4-Bromo-1-butanamine H20 and NaOH, which give
pyrrolidine. In hindsight I guess I should have looked at that and determined, but I also want to make sure and run it by people too.
Now "the book" does not, in fact, say anything about every specific SN2 reaction that can be dreamt up, and it doesn't say anything about
3-bromopropionic acid to gamma-butyrolactone. Why you should feel the need to point that out I dunno. Doesn't help this topic nor me or you. So anyway
to reference, should I go to http://pubs.acs.org/journal/joceah ?
Lastly Nicodem, we're all on different skill levels here when it comes to chemistry (and identifying jokes apparently), so perhaps it's good to
remember not everyone knows exactly what to do/where to go/who and how to ask. I used the search engine here. Can you find 4-bromobutanoic acid from
5-bromo-1-pentene or 3-Bromopropanoic acid to 3-Hydroxypropanoic acid in the search engine? I couldn't, hence the new topics.
I'm guessing now based on the info, 3-Bromopropanoic acid to 3-Hydroxypropanoic acid cyclizes first. So I'd need to use NaOH again to open the ring
back up. I'll reference if it's true, hopefully I can even find it...
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
'0' is not an element, you fool. Oxygen is, however, and has the symbol 'O'.
Quote: Originally posted by morry-cule  |
Lastly Nicodem, we're all on different skill levels here when it comes to chemistry (and identifying jokes apparently), so perhaps it's good to
remember not everyone knows exactly what to do/where to go/who and how to ask. I used the search engine here. Can you find 4-bromobutanoic acid from
5-bromo-1-pentene or 3-Bromopropanoic acid to 3-Hydroxypropanoic acid in the search engine? I couldn't, hence the new topics.
|
Our forum search engine is not very powerful, you might want to consider using Google instead: enter "Search term + sciencemadness". Other searches on
the general topic should be done too, to find the primary literature needed to identify procedures. If you come across a useful article or paper,
request it in the "References" section; if you haven't already, you will need to ask Polverone's permission for access.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by morry-cule  | Hey Nicodem, that response of mine pertaining H20 was a JOKE
...
4-Bromo-1-butanamine H20 and NaOH, which give pyrrolidine. |
Believing that the formula for water is H20 is not funny at all. Jokes are supposed to be funny, you know?
The empirical formula for water is H2O and its rational formula is HOH. Water is not H20: there is no known compound of twenty hydrogens bonding
between themselves!
| Quote: | | Now "the book" does not, in fact, say anything about every specific SN2 reaction that can be dreamt up, and it doesn't say anything about
3-bromopropionic acid to gamma-butyrolactone. |
I own you an apology. Sorry, but I automatically thought you were still trying to make GBL or GHB or whatever drug you were after previously, so I
merged the two threads. Without thinking about it, I thus missed that now you involved 3-bromopropanoic acid instead of 4-bromobutanoic acid. It is
the 4-bromobutanoic acid that gives gamma-butyrolactone. The 3-bromopropanoic acid would probably give a mixture of products.
I don't understand. You only linked to the latest TOC of the Journal of organic chemistry. Which article you refer to? Please use normal
citations for articles or give their DOI number.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Quote: Originally posted by Nicodem  |
I don't understand. You only linked to the latest TOC of the Journal of organic chemistry. Which article you refer to? Please use normal
citations for articles or give their DOI number. |
I believe he is referring to this journal in general.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
A joke doesn't have to be funny for everybody in order for it to be a joke. Otherwise there would be no jokes anywhere. I don't think Dane Cook's
funny, but that doesn't suddenly mean he doesn't make jokes as part of his profession.
H20 is a typo, you jokester, although a persistently strange one considering I typed NaOH without the zero error many times. =/
| Quote: | | I don't understand. You only linked to the latest TOC of the Journal of organic chemistry. Which article you refer to? Please use normal citations
for articles or give their DOI number. |
I don't understand either. I'm lost; organic chemistry has been somewhat of an overwhelming science for me.
| Quote: | | '0' is not an element, you fool. |
It could be a binary element in computing? ;D (j/k)
| Quote: | | Our forum search engine is not very powerful, you might want to consider using Google instead: enter "Search term + sciencemadness". Other searches on
the general topic should be done too, to find the primary literature needed to identify procedures. If you come across a useful article or paper,
request it in the "References" section; if you haven't already, you will need to ask Polverone's permission for access. |
Hmm. Good advice, thanks.
So does 3-bromopropanoic acid + NaOH + H2O cyclize into 2-oxetanone as the major product or are the bond angles too tight to work as
a cyclic ester?
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
Quote: Originally posted by morry-cule  | | So does 3-bromopropanoic acid + NaOH + H2O cyclize into 2-oxetanone as the major product or are the bond angles too tight to work as
a cyclic ester? |
No it does not, as long as you have the stuff in base it will be in it's open chain form. If you try traditional acid catalyzed esterification you'll
probably end up with polyesters. Yamaguchi esterification might be effective however...
[Edited on 7-1-2013 by kavu]
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by morry-cule  | | So does 3-bromopropanoic acid + NaOH + H2O cyclize into 2-oxetanone as the major product or are the bond angles too tight to work as
a cyclic ester? |
No.
It will quantitive yield 3-hydroxypropionic acid. If you don't belive check out: Justus Liebigs Annalen der Chemie, 1905 , vol. 342, p. 128
P.S.: Have vou ever heard from base catalysed ester synthesis in aqueous media? Me neither.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
| Quote: | | No it does not, as long as you have the stuff in base it will be in it's open chain form. If you try traditional acid catalyzed esterification you'll
probably end up with polyesters. Yamaguchi esterification might be effective however... |
| Quote: | No.
It will quantitive yield 3-hydroxypropionic acid. If you don't belive check out: Justus Liebigs Annalen der Chemie, 1905 , vol. 342, p. 128
P.S.: Have vou ever heard from base catalysed ester synthesis in aqueous media? Me neither. |
Pardon my inexperience, it just sounds odd that 4-bromobutanoic acid would give a lactone, as Nicodem was saying, but 3-bromopropanoic acid gives
3-hydroxypropionic acid when both are done with NaOH/H2O. Time to bury my face in my OChem textbooks again...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by morry-cule  | | Pardon my inexperience, it just sounds odd that 4-bromobutanoic acid would give a lactone, as Nicodem was saying, but 3-bromopropanoic acid gives
3-hydroxypropionic acid when both are done with NaOH/H2O. Time to bury my face in my OChem textbooks again... |
The 4-exo-tet cyclisations are allowed and known, but are very difficult to realize. In most cases intermolecular reactions are favoured over any
4-exo-tet cyclization. That's why it is unlikely much of the 3-bromopropionate would hydrolyse via the beta-lactone intermediate. As you can see in
the article you were pointed to, its hydrolysis gives a mixture of 3-hydroxypropionic acid and acrylic acid. This is an indication that there is no
neighbouring group assistance in the hydrolysis mechanism.
The 5-exo-tet cyclisations, on the other hand, are totally easy, hence substrates like 4-halobutanoates undergo hydrolysis via the lactone. Same thing
occurs during the nitrosation of 4-aminobutanoic acid.
The gamma-butyrolactone is in pH dependent equilibrium with gamma-hydroxybutanoate (UTFSE in regard to this topic). For this reason, it is not
possible to prepare pure 4-hydroxybutanoic acid, as it does not exist as such.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|