| Pages:
1
..
55
56
57
58
59
..
76 |
mesanaw
Harmless

Posts: 10
Registered: 29-10-2016
Member Is Offline
Mood: No Mood
|
|
First is the silver mirror from Tollen's reagent made in an old-fashion Coke bottle.

Some Mohr's Salt I made.

Copper Sulfate crystal still growing.

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
The disatvantages to work with molten Potassium in test tubes 

|
|
|
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Neme  | One picture is bunch of copper(II) acetate crystals.
In next pictures is my attempt to double phantom crystal, chrome alum crystal in chrome alum+ KAl alum mixture in KAl alum. The inner black crystal is
not visible tho so it's more like normal phantom crystal.
|
Did you do anything especial for the copper acetate crystals? I havent been able to crystalice it That way, un dark big crystals. All I get are lots
of small lighter ones. The biggest dark ones I got were like 2-3mm big. Probably because impurities, or temperature changes? I wanted to try making it
in a thermos, as I dont think I can get it much purer. I recrystaliced the acetate I have like 3 times in attempt to get crystals like those.
And since this is the pretty pictures topic, here is the result of my last attemp:

This one has copper wires fragments inside, because in one of the attemps I has the biggest (3mm) crystals grow con a copper wire, so I tried adding
some.
[Edited on 26-6-2017 by ficolas]
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Salt pyramids
Got these after concentrating a batch of sodium dichromate:
I'm suspecting it's sodium chloride (from the bleach). Hard to tell from the photos by they are hollow, inverted pyramids.

Another pic taken at an angle:

We're not banging rocks together here. We know how to put a man back together.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Inverted pyramids? They look like octahedra on the photo.
|
|
|
TheMrbunGee2
Harmless

Posts: 1
Registered: 16-6-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ficolas  |
Did you do anything especial for the copper acetate crystals? I havent been able to crystalice it That way, un dark big crystals. All I get are lots
of small lighter ones. The biggest dark ones I got were like 2-3mm big. Probably because impurities, or temperature changes? I wanted to try making it
in a thermos, as I dont think I can get it much purer. I recrystaliced the acetate I have like 3 times in attempt to get crystals like those.
[Edited on 26-6-2017 by ficolas] |
You should dissolve your acetate in warm water, filter it and let it evaporate. Cooling hot solution may not give large crystals..
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Because of poor solubility of copper acetate in water, it takes very long to grow big crystal of it. The key is to let the saturated solution
evaporate.
I made a bunch of saturated copper acetate solution by dissolving basic copper carbonate in acetic acid (BCC made from copper sulfate and sodium
carbonate). I keep adding the solution to container with seed crystals and letting it slowly evaporate.
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Today I recrystallized around 100g of erythritol from water, and over the course of a few hours some large and rather beautiful crystals grew.
Also I left a flask with methanol and dissolved boric acid in it on the bench for a few days. Upon cooling down most of the acid deposited on the
walls of the flask, but very slowly a thin layer of crystals formed, attached to the walls and suspended about 1 cm about the stir bar. The crystals
in this layer are in a branched pattern and look very cool.
Pictures: https://youtu.be/zbwy-PT3DUY
(I couldn't figure out how to resize them, I'll have to work on that)
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Hot off the press, a never-before-published photograph of plutonium tetrafluoride! This was obtained by nuclear anthropologist Martin Pfeiffer via
FOIA. It's a little disappointing (it is known as 'pink cake'; this is apparently misleading.) but upthread there are some very nice pictures of
exotic things like plutonium trichloride
If you're on twitter and interested in nuclear history/technology/sociology, definitely check him out!
<blockquote class="twitter-tweet" data-lang="en"><p lang="en" dir="ltr">BEHOLD INTERNET!<br><br>My 2nd novel contribution to
OSINT: my long sought pic of PuF4 'pink cake.' <br><br>FOIA'd for your enlightenment! <a
href="https://t.co/tnJqUhN5kc">pic.twitter.com/tnJqUhN5kc</a></p>— Martin Pfeiffer (@NuclearAnthro) <a
href="https://twitter.com/NuclearAnthro/status/883128593953378308">July 7, 2017</a></blockquote>
<script async src="//platform.twitter.com/widgets.js" charset="utf-8"></script>
[Edited on 7-7-2017 by mayko]
[Edited on 7-7-2017 by mayko]
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Selfmade Sodium Ferrate(VI) solution:

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
I have no clue what this is to be honest.
I was trying to react some Iodine with FeCO5 and thought this was an Iodine-Ethanol solution. Turns out and judging by the blue color this
was actually with an indicator, I guess starch? So this is the moment where the two chemicals meet but don't react as they are in different phases.

|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
An intermediate product in the synthesis I'm doing at work currently, made via a Wittig reaction.

Sure it's just a white solid, but I'm rather proud of the purity of it.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by zts16  | An intermediate product in the synthesis I'm doing at work currently, made via a Wittig reaction.
Sure it's just a white solid, but I'm rather proud of the purity of it. |
That looks like a disilyl ether of methyl 3,4-dihydroxycinnamate? It's hard to see the backend of the bottle being visible as well.
What would the IUPAC name be for this compound? The TBS addition throws me off.
What is in store for this compound? What is the intended purpose of those silyl groups?
[Edited on 28-7-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Lead picrate I made a week or so ago, as well as a demonstration of K3CrO8 with sulfur, and a few grams of black powder + magnesium I made for July
4th:



[Edited on 7/28/2017 by Velzee]
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Loptr  | Quote: Originally posted by zts16  | An intermediate product in the synthesis I'm doing at work currently, made via a Wittig reaction.
Sure it's just a white solid, but I'm rather proud of the purity of it. |
That looks like a disilyl ether of methyl 3,4-dihydroxycinnamate? It's hard to see the backend of the bottle being visible as well.
What would the IUPAC name be for this compound? The TBS addition throws me off.
What is in store for this compound? What is the intended purpose of those silyl groups? |
You're absolutely
right. I think it would be something like methyl 3,4-bis(dimethyltert-butylsilyloxy)cinnamate, though I normally abbreviate it if I have to write it
out. It's part of a series of reactions to make an anti-cancer drug, though I'm not sure if I can discuss the details publicly. The next reaction
though will be reduction to the analogous cinnamyl alcohol using DIBAl-H. The silyl groups were added prior to the Wittig reaction and are just there
as protecting groups. They will be removed at the end of the synthesis.
[Edited on 7-28-2017 by zts16]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
It seems like pharma is the only way to go if you want to do synthetic organic chemistry, so I might have to go with a grad degree in pharmaceutical
chemistry if I want to do that sort of stuff...looks fun though, zts16!
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Wet copper (II) chloride crystals, along with a small amount of excess HCl, giving rise to CuCl42- and the green color.

|
|
|
Morgan
International Hazard
    
Posts: 1662
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Sodium bentonite clay that had some "continental drift" due to evaporation. Not a pretty picture but if you did it again in a larger pan and let it
dry out, the image would be curious to see, with an artistic quality perhaps say if you had some separate in a long glass tube or something, the odd
shapes it might make?
When it was saturated the thixotropic clay made a faint resonant sound when the bowl was tapped, or more apparent you could feel a ring to it.

[Edited on 1-8-2017 by Morgan]
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Do drawings count?
A friend asked how cannabis plants make cannabinoids. I may have gone a little far in drawing my answer...
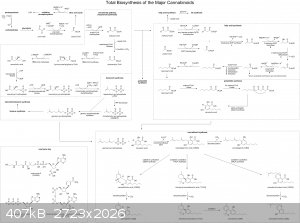
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Tollen's Reagent
I kinda went wild with Tollen's reagent...
(I hope to sell these at the local flea market as art)
   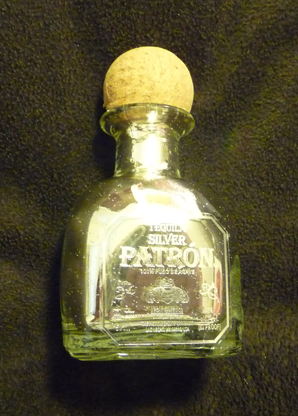                
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
These are amazing, what route and ratio did you use?
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by Supersonic  | Quote: Originally posted by Amos  | Copper(II) formate crystals. Their supernatant is the deepest of royal blues and yet the crystals are an icy-looking aqua color.
|
Large crystals have a nice form and beautiful color.
It`s a pity that they erodes even in warm air.
There crystals were synthesed from copper(II) hydroxide and formic acid.
|
Those are really beuatiful copper formate crystals. Especially, the second one is really big and it seems to be nicely shaped. What technique did you
use to grow it? I tried once but there was a problem with hydrolysis mostly and even in acidic environment the salt did not want to crystallize
easily. 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Ascorbic acid residue

Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
 
Nothing amazingly beautiful. Just some isopropyl propionate I made via Fischer esterification in my school's lab. 53% yield.
reality is an illusion 
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Image 1: preparing copper hydroxide from impure copper sulphate solution, the black is copper oxide formed when the hydroxide is heated above 80C.
Image 2: dissolving the subsequent copper oxide in dilute sulphuric acid, reminds me of the sea floor. 'Scuse the leg.
The reason I did this was to purify the copper sulfate, producing it from the metal using H2SO4 and KNO3 creates water soluble byproducts which are
removed by filtration.
H2SO4 + KNO3 -> KHSO4 + HNO3 {all aqueous}
Cu(s) + 4 HNO3(aq) -> Cu(NO3)2(aq) + 2 NO2(g) + 2 H2O(l)
Cu(NO3)2 + H2SO4 -> CuSO4 + 2 HNO3 {all aqueous}
Producing a solution containing CuSO4, KHSO4, and potentially residual KNO3
CuSO4(aq) + 2 NaOH(aq) -> Cu(OH)2(s) + Na2SO4
Cu(OH)2(s) + heat -> CuO(s) + H2O(l)
The alkali salts all remain in solution, and CuO is collected, readily reacting with acids unlike the base metal. The CuO can also be reduced to
copper powder, useful if you start with a single piece like pipe and desire a large surface area.
 
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
| Pages:
1
..
55
56
57
58
59
..
76 |