miamicanes
Harmless

Posts: 27
Registered: 30-9-2004
Member Is Offline
Mood: No Mood
|
|
opacity of glass, etc to UV-B and UV-A
How much UV-B and UV-A are blocked by normal (home depot-type) glass? How about lucite?
I'm considering building an enhancement to my current apparatus for oxidizing indigo blue into isatin using ozone + UV to take advantage of
natural sunlight when available. The basic idea I had was to direct the flow from the UV sterilizer to the shallow end of a long plastic paint roller
tray (raised so the surface tilts in the other direction), with the intent that it will flow down the tray (absorbing UV from sunlight) before
collecting at the bottom, where the pump will send it back into the ozonation + UV loop to make another trip. Since there are lots of potential
contaminants floating and blowing around outside, I want to enclose the sun-exposed surface in glass or lucite to protect the liquid from
contamination...
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Ordinary soda glass (for windows or bottles) blocks out UV light quite substantially. Plastics containing double bonds (including keto groups, as in
polycarbonate), would block out short UV light. The only transparent substance that is guaranteed not to block out UV light is fused silica, which is
used to make the sample cells for UV/visible spectrometry - expensive because of the high temperatures needed to melt silica without fusing with an
alkali. There may be some special glasses, containing a much higher proportion of silica than ordinary soda glass, which have satisfactory UV
transmission.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
How about something very thin, like platic wrap (used for food)? Mine is pure PE (or so it claims). PE has no double bonds, only single.
|
|
|
miamicanes
Harmless

Posts: 27
Registered: 30-9-2004
Member Is Offline
Mood: No Mood
|
|
Hmmm. How about the plastic used in clear flexible tubing (in particular, the kind used for aquarium tubing)? If that won't block too much, a
less-messy idea might be to simply make a big spiral from half-inch clear plastic tubing on a sheet of plywood and run the liquid coming from the UV
sterilizer into that, then dump it into the bucket from the other end of the spiral. That way, I'd avoid all contamination & evaporation and
give it at least another 5-10 seconds of hardcore UV exposure (Miami afternoon sun is pretty intense) on each trip through the loop...
Failing that, plastic wrap just might work 
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
http://www.miron-glas.com/
“If you want to find the secrets of the universe, think in terms of energy, frequency and vibration.” -Nikola Tesla
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
I wonder if the OP of this long dead thread ever bothered to search and study graphs of investigations done long ago?
http://www.ncbi.nlm.nih.gov/pubmed/19614895
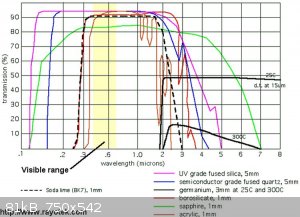
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|