underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
citric acid and its products
I was wondering if it is possible to do a tetranitrate salt of citric acid (Attachment) as it is going to have 4 hydroxyl groups.
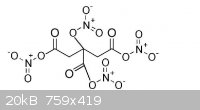
[Edited on 18-11-2013 by underground]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
I wonder if You can make the COOH to become C(NO2)3 by HNO3/H2SO4.
|
|
|
dangerdog
Harmless

Posts: 4
Registered: 15-11-2013
Location: Europe
Member Is Offline
Mood: Bi-Polar
|
|
The structure you have drawn is not a salt but the ester(s)
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Yes, you have right, i will correct it
[Edited on 18-11-2013 by underground]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
No, what you drew is not an ester. It's an acyl nitrate.
And also, I doubt you will be able to form a stable nitrate salt of (protonated) citric acid.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Cheddite Cheese  | No, what you drew is not an ester. It's an acyl nitrate.
And also, I doubt you will be able to form a stable nitrate salt of (protonated) citric acid. |
So any help should be really welcome
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Acyl nitrates are very unstable species and only exist in equillibrium with their hydrolosys products.
http://pubs.acs.org/doi/abs/10.1021/jo00912a020
In that paper, a mixture of acetic anhydride and 99% HNO3 were combined, and as a solution they behave like acetyl nitrate.
Since "citric anhydride" still has a hydroxyl and since water reverses the reaction, combined with the oxidizing nature of pure HNO3, I will venture
to guess it will be nearly impossible to prepare the tetranitrate ester of citric acid. Even if it was prepared, it would rapidly become unusably
hydrolyzed in air.
|
|
|
Boffis
International Hazard
    
Posts: 1837
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Citric acid only contains one OH group so I think that the best you can hope for is a mono-nitrate ester, which I have not heard of but check out
Beilstein and see if its there.
However, I don't hold out much hope because conc sulphuric acid converts citric acid to so called acetone-dicarboxylic acid (there are numerous
references to this on this web site and Org. Syn.) and fuming nitric acid oxidizes and nitrate the latter via a complex series of reaction into
furoxan-dicarboxylic acid or rather better the diethyl ester of acetone-dicarboxylic acid to the diethyl ester of furoxan-dicarboxylic acid (I can
provide quite a few reference here too if anyone is interested). The same product can be obtained by nitration ethyl acetoacetate. One has to wonder
what you would get nitrating triethyl citrate (OTC cosmetic ingredient)
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | Citric acid only contains one OH group so I think that the best you can hope for is a mono-nitrate ester |
What do you mean, i can see four
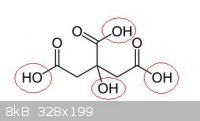
|
|
|
dangerdog
Harmless

Posts: 4
Registered: 15-11-2013
Location: Europe
Member Is Offline
Mood: Bi-Polar
|
|
There is only 1 OH group, the others are carboxylic acids.
|
|
|
Boffis
International Hazard
    
Posts: 1837
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thank you Dangerdog! At least there one switched on chemist out there.
The "OH" on the carboxylic acid groups act as a discrete unit, as though the two oxygens are identical; presumably the hydrogen is free to move
between two.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Really interesting, i did not know that, so you have right, there is only one OH group that you can nitrate!!
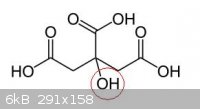
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Dangerdog it looks like there are several bonds that they can be nitrated except OH groups, like from toluene to trinitrotoluene, but why only those 3
C-H groups can be nitrated and the rest of them not ?
Also what else kind of ponds can be nitrated ?
[Edited on 20-11-2013 by underground]
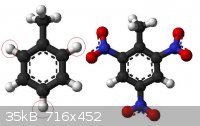
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by underground  | Dangerdog it looks like there are several bonds that they can be nitrated except OH groups, like from toluene to trinitrotoluene, but why only those 3
C-H groups can be nitrated and the rest of them not ?
Also what else kind of ponds can be nitrated ?
[Edited on 20-11-2013 by underground] |
It can,
http://en.wikipedia.org/wiki/Hexanitrobenzene
C6H3(NO2)3 → C6H3(NHOH)3 (partial reduction)
C6H3(NHOH)3 → C6(NO2)3(NHOH)3 (nitration)
C6(NO2)3(NHOH)3 → C6(NO2)6 (oxidation)
However you need different routes.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by DubaiAmateurRocketry  | Quote: Originally posted by underground  | Dangerdog it looks like there are several bonds that they can be nitrated except OH groups, like from toluene to trinitrotoluene, but why only those 3
C-H groups can be nitrated and the rest of them not ?
Also what else kind of ponds can be nitrated ?
[Edited on 20-11-2013 by underground] |
It can,
http://en.wikipedia.org/wiki/Hexanitrobenzene
C6H3(NO2)3 → C6H3(NHOH)3 (partial reduction)
C6H3(NHOH)3 → C6(NO2)3(NHOH)3 (nitration)
C6(NO2)3(NHOH)3 → C6(NO2)6 (oxidation)
However you need different routes. |
This is benzene, not toluene
What about octonitronaphthalene
[Edited on 20-11-2013 by underground]
|
|
|