12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
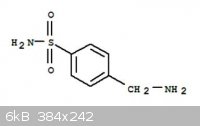
Synthesis of marfanil from toluene
I found this chemical in Organic chemistry made simple and worked out a synthesis route from toluene. Marfanil looks like this (its not
particularly common).
My route was
1. C6H5CH3 + H2SO4 -> p-C6H4CH3SO3H +H2O
2. C6H4CH3SO3H + PCl5 -> C6H4CH3SO2Cl +POCl3
3. C6H5CH3SO2Cl + NH3 -> C6H5CH3SO2NH2 + HCl
4. C6H4CH3SO2NH2 + Cl2 -> C6H4CH2ClSO2NH2 +HCl
5. C6H4CH2ClSO2NH2 + NH3 -> Marfanil + HCl
Steps 4&5 could be done first (does this need to be done for ease of synthesis?) but otherwise any other suggestions?
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<ol type="1" start="1"><li>C<sub>6</sub>H<sub>5</sub>CH<sub>3</sub> +
H<sub>2</sub>SO<sub>4</sub> → <a href="http://en.wikipedia.org/wiki/P-Toluenesulfonic_acid"
target="_blank"><em>p</em>-TsOH</a> <img src="../scipics/_wiki.png" /> + H<sub>2</sub>O</li>
<ul><li><a href="http://library.sciencemadness.org/library/books/vogel_practical_ochem_3.pdf#page=552">Vogel</a> <img
src="../scipics/_pdf.png" /></li><li><strong><a href="viewthread.php?tid=17783">Unexpected problems with p-TsOH
synthesis</a></strong></li><li><strong><a href="viewthread.php?tid=11520">alteration of Vogel p-toluenesulfonic
acid syn</a></strong></li><li><strong><a href="viewthread.php?tid=21363">Sodium p-toluenesulfonic
acid</a></strong></li><li><strong><a href="viewthread.php?tid=3132">Toluenesulfonic acid and Sulfonic acids in
genera</a></strong></li></ul>
<li><em>p</em>-TsOH + <a href="http://en.wikipedia.org/wiki/Phosphorus_pentachloride"
target="_blank">PCl<sub>5</sub></a> <img src="../scipics/_wiki.png" /> → <a
href="http://en.wikipedia.org/wiki/4-Toluenesulfonyl_chloride" target="_blank"><em>p</em>-TsCl</a> <img
src="../scipics/_wiki.png" /> + <a href="http://en.wikipedia.org/wiki/Phosphoryl_chloride"
target="_blank">POCl<sub>3</sub></a> <img src="../scipics/_wiki.png" /></li>
<li><em>p</em>-TsCl + NH<sub>3</sub> → <em>p</em>-TsNH<sub>2</sub> + HCl</li>
<ul><li><a href="http://www.organic-chemistry.org/synthesis/N1S/sulfonamides.shtm" target="_blank">Sulfonamide syntehsis by S-N
coupling</a> <img src="../scipics/_ext.png" /> (Org. Chem. Portal)</li><li><a
href="http://www.chemspider.com/Chemical-Structure.6033.html" target="_blank">4-Toluenesulfonamide</a> <img src="../scipics/_ext.png"
/> (ChemSpider/RSC)</li></ul>
<li><em>p</em>-TsNH<sub>2</sub> + Cl<sub>2</sub> → C6H4CH2ClSO2NH2
+HCl</li>4-(chloromethyl)benzenesulfonyl chloride
<li>C6H4CH2ClSO2NH2 + NH<sub>3</sub> → Marfanil + HCl</li></ol>
<em>Welcome to Science Madness!</em>
[editing]
[Edited on 1.1.14 by bfesser]
[Edited on 1.1.14 by bfesser]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Steps 3 and 5 could probably be combined at the end.
[Edited on 1-1-2014 by Cheddite Cheese]
|
|
|
eidolonicaurum
Hazard to Self
 
Posts: 71
Registered: 2-1-2014
Location: Area 51
Member Is Offline
Mood: Hydric
|
|
Could steps 1 and 4 be combined in some way?
|
|
|
orville redenbacher
Banned
Posts: 10
Registered: 13-11-2014
Member Is Offline
Mood: No Mood
|
|
I thought that since carbon must have 4 bonds and nitrogen must have 6, this isn't a real chemical
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Carbon
with a formal charge of zero has 8 valence electrons, often forming 4 sp3 sigma bonds or some other hybridization thereof.
I believe your confusion may stem from the implicit lone pair on nitrogen, since it has 6 bonding electrons here, with one lone pair, giving
it sp3 hybridization, the same as a carbon with a full valence of 8 bonding electrons.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
it would be better to first halogenate the sp3 carbon using TCCA or passing chlorine through boiling toluene and then sulphonating the
ring
you could also brominate it instead of chorination
the mechanism for benzylic halogenation is a free radicle one.
so if an electron withdrawing group like HSO3 is first put in the ring ,it will destabilize the radicle formed while the benzylic
halogenation is being carried out
http://www.masterorganicchemistry.com/2013/08/05/what-factor...
then PCl3 could be used to make it para chloromethyl chloro sulphonic acid
if you treat the compound with ammonia directly ,wouldn't it run away to give a tertiary amine instead of primary amine
you will get only primary amine if you do delepine reaction as hexamine is very easy to make
http://en.wikipedia.org/wiki/Del%C3%A9pine_reaction
alternatively ,you could do benzylic hydroxylation using SeO2
http://pubs.acs.org/doi/abs/10.1021/jo00393a045
then sulphonate the benzyl alcohol and then treat with PCl3 to convert both the alcoholic groups(one on the sp3 carbon and the other of the
sulpho group) to chloride
but do you think treating benzyl alcohol with sulphuric acid would form benzyl hydrogen sulphate, in the same way as ethyl hydrogen sulphate is formed
when ethanol is treated with sulphuric acid
also i realize now that if the halogenation of the sp3 carbon is done first, followed by sulphonation ,is there any chance that the chlorine on the
sp3 carbon would be replaced by OH to form para hydroxymethyl sulphonic acid
[Edited on 16-11-2014 by CuReUS]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Allylic reactions don't necessarily imply that benzylic cognates are interchangeable. If you look into the mechanism you cited for selenium dioxide
oxidations, you will see that the allylic mechanism isn't feasible in a benzylic system, which is likely why there are no examples in the paper you
mentioned, nor that I am familiar with in my interest of related Riley oxidations.
This is substantiated in the thesis cited immediately below, where toluene was unreactive to selenium dioxide oxidation, as expected given the
mechanism now known.
Transactions of the Kansas Academy of Science 96 (3-4), 1993 pp167-180.
12thealchemist has a feasible looking synthesis and, if interested in the synthesis itself rather than just the "on paper" exercise,
can minimize polyalkylation with good stirring, temperature control, and the slow addition of halogenated compound to excess amine. Not ideal, but
possibly less hassle than a Delepine or Gabriel reaction.
If interested in actual synthesis rather than theoreticals, please see page 525 of Kar's Medicinal Chemistry for a 4-amino-methyl-benzene sulphonamide
(Mafenide) industrial synthesis. If you are interested in a newer electrochemical synthesis, DOI: 10.1002/hlca.200690123 might be of interest.
[Edited on 25-11-2014 by Chemosynthesis]
|
|
|