underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
protonatation/deprotonation of molecules
There is a way to protonate or deprotonate the amine group of some molecules to forms salts. The idea is to protonate/deprotonate some molecules to
form denser salts for better performance and OB. But how it can be done actually ?
For example i was thinking to deprotonate a molecule (like nitroguanidine) making it to behave like an anion (-1), Then with an aluminum cation (+3),
add 3 molecules of nitroguanidine to it to make the aluminum nitroguanidine salt with 1 aluminum molecule and 3 nitroguanidine molecules, i guess it
will be powerful. On the other hand, protonation of nitroguanidine can form salts like nitroguanidine nitrate / perclorate e.t.c.
[Edited on 20-2-2014 by underground]
|
|
|
Metacelsus
International Hazard
    
Posts: 2532
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It's not that easy. Deprotonation of guanidine will create an extremely strong base, as guanidine is a base already. The pKa will be around 35.
Protonation is much easier.
How would you plan to prepare the aluminum salt? It will not be easy. You would probably need aluminum hydride, and even that may not work.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
What about nitroguanidine, as i said above ?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
nitroguanidine seems much easier.
I recently got interested into Dinitrourea and dinitroguanidine's salts. I wonder if the hydroxylammonium salt of them exist since the ammonium salt's
density is pretty horrible. Hydroxylammonium would give much better oxygen balance, density, and hydrogen bonds at same time.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by DubaiAmateurRocketry  | nitroguanidine seems much easier.
I recently got interested into Dinitrourea and dinitroguanidine's salts. I wonder if the hydroxylammonium salt of them exist since the ammonium salt's
density is pretty horrible. Hydroxylammonium would give much better oxygen balance, density, and hydrogen bonds at same time. |
That is true, but in practice how you can do that ? protonane and deprotonate molecules ?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by underground  | Quote: Originally posted by DubaiAmateurRocketry  | nitroguanidine seems much easier.
I recently got interested into Dinitrourea and dinitroguanidine's salts. I wonder if the hydroxylammonium salt of them exist since the ammonium salt's
density is pretty horrible. Hydroxylammonium would give much better oxygen balance, density, and hydrogen bonds at same time. |
That is true, but in practice how you can do that ? protonane and deprotonate molecules ? |
I am not an expertise in chemistry. However if you shows me a structure of an molecule, I could tell you which hydrogen is acidic and can form salts.
Guanidine it self is hard be an anion and the reason has already been explained by Cheddite Cheese. When a compound have a lot of electron withdrawing
group(for EM nitro group is most common), then the polar bonded hydrogen atoms could be deprotonated.
[Edited on 20-2-2014 by DubaiAmateurRocketry]
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I am talking about nitroguanidine and not Guanidine. Guanidine indeed it is a strong base
|
|
|
DraconicAcid
International Hazard
    
Posts: 4280
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Well, the Merck Index doesn't give a pKa for nitroguanidine (and googling gives a number of widely varying values), but it does say it is soluble in
aqueous alkalis (but not carbonates). This suggests that it can be deprotonated in aqueous solution, and reaction of this solution with a metal salt
*may* give precipitation of the metal nitroguanidate(?). I wouldn't bother trying aluminum, though, since that would most likely give a stubborn gel
of aluminum hydroxide. A transition metal with an affinity for nitrogen may work better.
This is purely theoretical, of course- I have no experience with nitroguanidine.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
It is really difficult to find any information for this chemical, google does not have almost nothing about that.
Nitroguanidine nitrate (NGN) it is said that it is more powerful than HMX!! With a little search, in this topic, the last one post, it is said:
Quote: Originally posted by VladimirLem  |
I read about DiNitroGuadinine and NitroGuadinineNitrate and both can be made by the nitration of nitroguanidine (NGN needs only 60%
HNO3 and DNG needs 100%+H2SO4/oleum)...but i dont want to waste such many H2SO4 on DNG and dont want to make oleum (to expensive and
much much work - and fucking dangerous btw)
other question, there is not much information about NGN...only some translation that says it would be more powerful than DNG or
ammoniumdinitroguanidine...can this be? |
Also an another post form Ral123 about NGN
Quote: Originally posted by Ral123  | Nitroguanidine nitrate-the legend says it's more powerful then HMX and it's the poor man's elite explosives equivalent. Density about 2, velocity
about 9300 and a guy has ripped a whole in a concrete wall with some of it  If
it wasn't acidic and with doubtful storage stability, I'd be making some for blast cap base charges and mini boosters. If
it wasn't acidic and with doubtful storage stability, I'd be making some for blast cap base charges and mini boosters. |
[Edited on 21-2-2014 by underground]
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Nitroguanidine nitrate may be powerful, but it's not very stable. It slowly loses HNO3 in contact with air.
It's made by dissolving nitroguanidine in hot, concentrated nitric acid and allowing it to cool.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Dornier 335A  | Nitroguanidine nitrate may be powerful, but it's not very stable. It slowly loses HNO3 in contact with air.
It's made by dissolving nitroguanidine in hot, concentrated nitric acid and allowing it to cool. |
Too bad
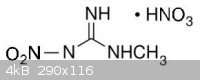
I guess this is because of its low basicity due to nitro group. That is why i guess the aminonitoguanidine nitrate is more stable, cause of its amino
group, it becames a stronger baze than nitroguanidine.
An another idea, As long as the formation of aminonitroguanidine needs hydrazine, a chemical that i really want to avoid, i was wondering if the amino
group can be replaced with other group like methyl group for something like methylnitroguanidine nitrate.
[Edited on 21-2-2014 by underground]
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
well....what if someone would react NGN with stuff like ammonia or urea ?
would the NGN decomposite to like NG and AN/UN or could that bring a bit more stability and would make the OB of NGN (OB +5%) to nearly zero
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
underground, it is CH3 on instead of CH there.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Yea that is true... i forget the number, i will fix it 
Quote: Originally posted by VladimirLem  | well....what if someone would react NGN with stuff like ammonia or urea ?
would the NGN decomposite to like NG and AN/UN or could that bring a bit more stability and would make the OB of NGN (OB +5%) to nearly zero
|
I bet AN/UN will be formed...
[Edited on 21-2-2014 by underground]
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
Quote: Originally posted by underground  |
Yea that is true... i forget the number, i will fix it 
Quote: Originally posted by VladimirLem  | well....what if someone would react NGN with stuff like ammonia or urea ?
would the NGN decomposite to like NG and AN/UN or could that bring a bit more stability and would make the OB of NGN (OB +5%) to nearly zero
|
I bet AN/UN will be formed...
[Edited on 21-2-2014 by underground] |
damn 
and how about my question you quoted from the other thread - does it seem possible to make first NGN with (60% hno3) and then making DNG with only
95<% HNO3 and maybe a little bit H2SO4? (2 step instead of one step-nitration the same way it works at RDX over HDN)
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by VladimirLem  |
damn 
and how about my question you quoted from the other thread - does it seem possible to make first NGN with (60% hno3) and then making DNG with only
95<% HNO3 and maybe a little bit H2SO4? (2 step instead of one step-nitration the same way it works at RDX over HDN) |
Yea it will may be possible but maybe the yelds will not going to be so high
I have also an other question too, adding h2so4 to aminoguanidine nitrate will it yeld to aminonitroguanidine ?
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
Quote: Originally posted by underground  | Quote: Originally posted by VladimirLem  |
damn 
and how about my question you quoted from the other thread - does it seem possible to make first NGN with (60% hno3) and then making DNG with only
95<% HNO3 and maybe a little bit H2SO4? (2 step instead of one step-nitration the same way it works at RDX over HDN) |
Yea it will may be possible but maybe the yelds will not going to be so high
I have also an other question too, adding h2so4 to aminoguanidine nitrate will it yeld to aminonitroguanidine ? |
i have two synthesis files, and both use the Nitroguanidine+hydrazine-way
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I guess there is no other way...
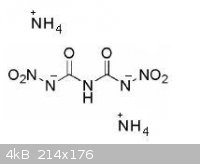
I have also an another more promised idea
Dinitrobiuret itself has a positive OB and a VoD of 8660m/s
Also it can easily give 2 of his hydrogen molecules to any base with a -2 charge. So a diammonium dinitrobiuret can be exist, and i bet it will be
really stable with a really high VoD.
[Edited on 21-2-2014 by underground]
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Guanidine nitroformate is another one. It has a detonation velocity like RDX and low impact sensitivity. It can be made by combining a dilute
guanidine solution or guanidine carbonate with trinitromethane. OB is -7.6%.
|
|
|
underground
National Hazard
   
Posts: 698
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
They usually do the use of zydrazine because i believe theu want to avoid the long way below for making aminonitroguanidine. Also i can not see any
reason why this is not going to work
nitroguanidine --(acetic acid+zink)--> aminoguanidine bicarbonate
aminoguanidine bicarbonate --(15% nitric acid)--> aminoguanidine nitrate
aminoguanidine nitrate --(H2SO4)--> aminonitroguanidine
aminonitroguanidine --(nitric acid)--> aminonitroguanidine nitrate
And all of these without any exotic chemicals and dilute nitric acid!!
[Edited on 22-2-2014 by underground]
|
|
|