Hey There
Harmless

Posts: 13
Registered: 20-3-2014
Location: Washington
Member Is Offline
Mood: Good
|
|
May you spare some time for a "newbie" question?
Hey there!
So first off, I have very little training in chemistry, but lately (more like last year) I've found it very interesting, so interesting in fact, I
reconsidered going to med school since 1. I LOVE human anatomy And 2. I'll have Orgo to coincide! So anyways i'm excited, but to my question... What
reactions and reaction conditions would have to take place for Trazodone to be reduced to mCPP, and what mechanisms would be taking place during the
reactions?
Now since I'm sure some of you know what this is (if you dont, there you go: http://en.wikipedia.org/wiki/Meta-Chlorophenylpiperazine), and before anyone gets their panties in a bunch, I DO NOT PLAN ON ATTEMPTING ANYTHING
ILLEGAL, and I'm not saying that to stay away from incrimination and/or criticism. If you read up on mCPP its effects sound risky, uncomfortable and
dull so being high on life sounds like a more suitable option.
I'm just curious after I read that our bodies metabolize Trazodone too mCPP, then I saw the similarities in their molecules, and it sparked an
interest. Anyhow, I'd appreciate any of your thoughts and knowledge about Chemistry, there's a perfect picture I found online below.
Sincerely,
Jennifer (aka Hey There)
By the way, here's a link to Trazodone in case you don't know what that is either: http://en.wikipedia.org/wiki/Trazodone
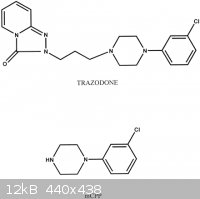
[Edited on 20-3-2014 by Hey There]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Hi. First, I'd like to mention that mCPP is not currently scheduled in your location anyway, though I find it highly unlikely anyone would want to
synthesize such a compound for attempted profit or recreation, much less from prescription Trazodone.
Are you more interested in the biological relevance or the synthetic aspect of the chemistry? I ask because they often seem radically different. I'm
not sure how you'd practically cleave the propyl linker group in a biomimetic reaction without touching the substituted phenyl, but enzymatically, you
can stabilize various parts of a pharmacological ligand through hydrogen bonding and conformational constraints. If I had to speculate, maybe you
could nitrosate with one equivalent of nitrite in acid, separate the secondary mCPP nitrosamine, then hydrolyse in base.
I have more than one citation available, but this demonstrates how messy I expect that to be:
http://dx.doi.org/10.1016/S0040-4039(00)88318-5
I forget how to do it, but webMO will let you run a simple chemical simulation in Gaussian, GAMESS, or MOPAC to see what bonds were most likely to
cleave first.
In organisms, this is an oxidative deamination, and the reaction falls under a category of reactions known as phase 1 metabolism. I would speculate
that the cysteine linked cytochrome oxidase P4503A4 (CYP4503A4) heme oxidizes the methylene carbon alpha to the piperazine nitrogen, yielding an
aminoalcohol which is cleaved to the amine and aldehyde for further metabolism, though there are other metabolic possibilities.
It appears as though as of at least 2010, no force field parameters for the binding pocket of the relevant metabolic enzymes weren't published. I
can't point you to a proposed mechanism for trazodone, but it appears to primarily be metabolized to mCPP through CYP4503A4, as noted on Wikipedia http://dmd.aspetjournals.org/content/26/6/572.full.
If you really wanted to try to get an idea for how this xenobiotic is metabolized, I would say spend an inordinate amount of time and look into Moore
et al.'s docking work on autodock tools (ADT) below, perhaps compare with VINA (not sure which would be more accurate in this instance), then use that
as a basis for some kind of molecular dynamics simulations. I'm not sure if ADT or VINA can do MD sims, but Molecular Operating Environment (MOE)
would probably be your best bet, as it's great software, and unfortunately not free. You might be able to glean potential mechanisms from the nearest
residues.
DOI:10.1016/j.cplett.2005.12.015
PMC2958526
DOIi: 10.1098/rstb.2012.0434
|
|
|
|