deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
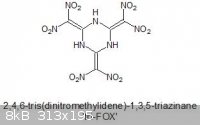
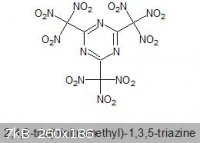
tri-FOX
I trust everyone is well familiar with FOX-7.
I was wondering if the trimeric version has ever been synthesised which should be significantly more energetic, what with a higher density and better
OB.
It's IUPAC name would be 2,4,6-tris(dinitromethylidene)-1,3,5-triazinane, but I like the name tri-FOX 
A cursory search did not turn up much, but I did find a somewhat related compound on a Wiki stub, oddly enough:
2,4,6-Tris(trinitromethyl)-1,3,5-triazine
Citing for that compound:
"Synthesis of 2,4,6-Tris(trinitromethyl)-1,3,5-triazine", Alexey V Shastin, Tamara I Godovikova, Svetlana P Golova, Vladimir S Kuz'min, Lenor I
Khmel'nitskii, Boris L Korsunskii, Mendeleev Communications; Volume 5 (1995), Number 1, Pages 17–18.
"Nucleophilic Substitution Reactions of 2,4,6-Tris(trinitromethyl)-1,3,5-triazine. 3. Reaction of 2,4,6-Tris(trinitromethyl)-1,3,5-triazine with
Azides and Hydrazine", A. V. Shastin, T. I. Godovikova and B. L. Korsunskii. Chemistry of Heterocyclic Compounds, March 2003; 39(3): 354-356.
Okay, so thoughts and info on tri-FOX?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
If it could be synthesized, I think the 3 NH are acidic and we should be able to make some interesting salts out of them. I do believe this compound
could have a density around 2g/cm3 and should possess higher explosive performance than HMX and also less sensitive due to the hydrogen bonds from NH.
But the synthesize cost probably makes it hard to progress just like many others.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Agreed, not an easy make and I don't even have speculations for this one, but DAMN it's sexy 
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Compared to this, I think 5-(Dinitromethylene)-4,5-dihydro-1H-tetrazole have more potential however is even harder to synthesize. the 2 hydrogen is
acidic and dianion salts could be synthesized although only the dihydrazinium was made.
This structure of this compound is very similar to FOX7. Just like how 5-aminotetrazole is just 2 extra nitrogen instead of 2 hydrogen in guanidine
forming a ring, this compound is exactly the same but analogous to FOX7.
From this paper. http://pubs.acs.org/doi/abs/10.1021/ic400919n
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
the two molecules posted here have been studied theoretically 10 years ago and published in a conference paper.
See:
Shastin, Alexey V.; Godovikova, Tamara I.; Pivina, Tatyana S.; Surikova, Yulia N.; Golova, Svetlana P.; Vorontsova, Svetlana K.; Korsunsky, Boris L.,
Structure and energy content study of three-substituted 1,3,5-triazines and their hexahydrotriazine isomers by
computer chemistry methods, 35 th International Annual Conference of ICT (2004).
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks Dany... another piece of the puzzle. I like the fact we are collecting info about this and putting it up here, seems an
excellent first step into someday cracking this nut.
It is interesting to note that 2,4,6-tris(trinitromethyl)-1,3,5-triazine (which has been successfully synthesised, see opening references on first
post) could hypothetically be partially reduced to tri-FOX. Perhaps the same 'trick' to partially reducing tetranitromethane with hydrogen peroxide
to form nitroform could be employed here since 2,4,6-tris(trinitromethyl)-1,3,5-triazine is an oxidant.
From https://sites.google.com/site/ecpreparation/nitroform:
| Quote: | Hantzsch and Rinckenberger obtained the ammonium salt of trinitromethane by treating tetranitromethane with aqueous ammonia.
It is well known that tetranitromethane can be reduced to nitroformate salts using an alkaline solution of hydrogen peroxide. This is the most usual
route for preparing trinitromethane. Prepare a solution of 168 g of potassium hydroxide in 350 mL of water in a round-bottomed 1000-mL Florence flask,
and cool to 5 °C with a salt-ice bath. While stirring, add 108 mL of 30% hydrogen peroxide to the solution. Next, add 117 mL of tetranitromethane at
a rate which keeps the temperature at 20-25 °C, add while stirring. The temperature is then allowed to rise to 30 °C over 15 minutes. The bright
yellow solid, that should have formed, is filtered to collect it using glass filter paper because of its high acidity, washed with anhydrous methyl
alcohol, then anhydrous ethyl ether, and finally air dried to give 100% of the potassium salt of trinitromethane. The salt is suspended in anhydrous
ethyl ether and anhydrous hydrogen chloride gas is passed in until the yellow color disappears. The white precipitate of potassium chloride is
filtered off and washed with anhydrous ethyl ether. The ethyl ether is evaporated from the filtrate and additional washings at reduced pressure give
85-90% of crude trinitromethane which can be purified by sublimation.
Although usually an oxidizer, in some reactions hydrogen peroxide can act as a reducing agent. For example, it reacts with hypochlorite to form
chloride and oxygen gas. Similarly, an alkaline solution of H2O2 reduces Cl2 to Cl- ions. |
The problem is I cannot see the full reference anywhere for this snipped specifically  , however, in the preceding paragraph they reference: , however, in the preceding paragraph they reference:
Hantzsh, Chemische Berichte, 39, p2478, (year 1906)
Are we to assume that this is from there as well?
Anyhow, as a first guess, the synthetic approach to preparing tri-FOX may be to synthesise 2,4,6-tris(trinitromethyl)-1,3,5-triazine by published
methods and then selectively reducing it, for example by a modified peroxide method employed for tetranitromethane.
As Dubai et al alluded, tri-FOX is probably acidic, hence it may well form as a salt if a base is employed for the reduction
(probably a must).
Again, this is speculative, but at least we're gathering information on known chemistry and trying to build from that.
[Edited on 24-4-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
You might be onto something very interesting.
As other members mentionned your putative molecule is acidic; what might be good news to make heavy metals salts primaries, or energetic amine salts
(NH3, NH2OH, NH2-NH2, ...).
Despite its theorical predicted unstability (due to loss of aromaticity and presence of dinitromethyl moeities); there are possible resonance
structures that makes it look like triphenylene. Protons can switch from one place to another; so your molecule has actually 3 possible structure
interchangeable and in equilibrium.
The strongest part comes from the tri-anion of that molecule that should display a molecular orbital fully delocalised and planar like a polycyclic
aromatic hydrocarbon would be...this is very good for density increase by molecular packing.
All bonds are partly sigma (sp3) and partly pi (sp2) so the bonds lengths should be between double (short) and single (long).
Hereunder you may find an image of what I just explained.
-The green stars show possible H bondings between acidic H's (from nitronic moieties) and N's free electronic doublets (of triazine); or between
acidic H's (from isocyanuric moieties) and O's free electronic doublets of the nitro groups.
-The two extreme structures comes very similar to triphenylene.
-The blue curves (should be dotted lines...but it was hard enough to draw  )
represent the delocalised 3 électrons all over the planar molecular structure just as if it was aromatic. )
represent the delocalised 3 électrons all over the planar molecular structure just as if it was aromatic.
-A third double arrow should link the first molecule and the last.
-For convenience of drawing nitro groups they were not all in spreaded writting. But you must keep in mind that -NO2 is planar -N(=O)2 with an angle
of 120° between -N, N=O and N=O'.
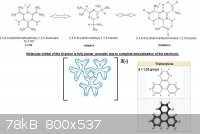
Note the extremely high density of triphenylene what is a simple hydrocarbon (1,30 g/ccm vs 0,88 g/ccm for benzene).
Note also the density increase between benzene, pyridine and diazines due to substitution of aromatic C-H (13 units of atomic mass) by a nitrogen (14
units of atomic mass):
*Benzene d = 0.88
*Pyridine d = 0.98
*Pyrimidine (1,3-diazine) d = 1.02
*Pyridazine (1,2-diazine) d = 1.11
*Pyrazine (1,4-diazine) d = 1.03
So in our structure triphenylene-like due to the presence of many nitrogens, the density will be high; not only because of the triazine core, but also
because of the other nitrogen and oxygen from the nitro-groups involved in the molecular orbital.
Density will be high for the protonated molecule but even more for its tri-metalic or triamine salts.
[Edited on 29-4-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Excellent commentary PHILOU and thanks, I had not thought of the tautomers of this compound nor of the aromaticity of the trianion...
this is very interesting.
BTW, I liked your blue aura of delocalisation 
As you and Dubai et al mentioned, the salts would be very interesting/powerful primaries.
So far the most realistic synthetic route I could come up with would be a partial reduction of the known oxidant mentioned in the opening post under
basic conditions, so the salt formation would be unavoidable, probably as the potassium salt, K3C6N9O12, if one employed a potassium phosphate buffer
for example, with hydrogen peroxide as reducing agent?
I would guess that such salts would not be very water soluble, but that is admittedly, highly speculative.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
FOX can't be done via reaction of 1,1-dichloro-2,2-dinitroethene (DCDNE) with amonia (it produces NH4C(C#N)(NO2)2 the ammonium salt of
cyanodinitromethane) but maybe can DCDNE react with urea?
Cl2C=C(NO2)2 +2 NH2-CO-NH2 --> (H2N-CO-NH)2C=C(NO2)2. 2 HCl (chlorhydric acid on the terminal NH2).
Cl2C=C(NO2)2 + NH2-CO-NH2 --> H2N-CO-NH-C(Cl)=C(NO2)2. HCl (chlorhydric acid on the terminal NH2).
This later could trimerise in a related molecule close to the desired tri-FOX:
3 H2N-CO-NH-C(Cl)=C(NO2)2. HCl --> (-C(=C(NO2)2)-N(-CO-NH3Cl)-)3
(-C(=C(NO2)2-N(-CO-NH3Cl)-)3 -H2O-> (-C(=C(NO2)2)-NH-)3 + 3 CO2 + 3 NH4Cl
I have done the nitration of trichlorethylene what is known to produce Cl2C=CCl(NO2)... this product is a heavy yellow oïl with a pungnant smel like
chloropicrine and it reacts with amonia to substitute at least one chlorine atom by an amino group.
The product is easily done by simple mixing of HNO3 69% with trichlorethylene (trichlorethene) with moderate heating.
Maybe reacting DCDNE with FOX could be another way:
Cl2C=C(NO2)2 + (H2N)2C=C(NO2)2 --> (-NH-C(=C(NO2)2)-)n + tri-FOX
Another possible way, due to the tautomeric forms identified, is via cyanuric tri-halides made from cyanogen halide trimerisation:
3 Cl-C#N --> (-C(Cl)=N-)3 (works also with Br-C#N and I-C#N)
This is the tri-chloride of cyanuric acid.
CTC is known to be subject of easy chlorine atome exchange:
Cyanuric triazide is made by mixing cyanuric tri-chloride with sodium azide in dimethylformamide (DMF).
Cyanuric tri-nitroformiate (2,4,6-Tris(trinitromethyl)-1,3,5-triazine) is made also by methatesis between cyanuric tri-halide and a nitroformiate
(trinitromethanate) salt.
Based on this, one might assume, that reacting cyanuric trihalide (chloride, bromide or iodide) with a dinitromethanate salt (Li, Na, K, Ag, Hg?) in a
good solvent for such substitutions (DMF, DMSO) will lead to the desired tri-FOX
(-C(X)=N-)3 + 3 CH(NO2)2(-) --> (-C(-CH(NO2)2)=N-)3 + 3 X(-).
X = Cl, Br, or I
So they are other possible ways probably easier than yours.
About the aromaticity of the tri-FOX; in one of your references (I think it is the website of AndersHoveland) if you follow the link about dinitromethyltetrazole you also see the -NH-C(=C(NO2)2)-NH- structure and it displays the same propertie of planar aromaticity and electron
delocalisation all over the molecule:
Quoted from reference:
"The structure of dinitromethyl tetrazole can be written
N4H2C=C(NO2)2
and contains a pentagonal ring composed of four nitrogen atoms and one carbon atom. The actual structure of the molecule is more difficult to write,
as it is fully aromatic [delocalized bonding]. I think it was mentioned somewhere that it forms yellow crystals that are fairly acidic."
Edit:
In the case the methatesis reaction of the dinitromethanate doesn't work well...there is a chance it reacts as the aci-nitro-form (thus bridging
substitution occurs via the oxygen atom and not the C atom) and not the nitro-form (conventional C bonding); one could also try via MeC(C#N)(NO2)2 the
metalic salt of cyanodinitromethane with Me = Li, Na, K, Ag, Hg?) in a good solvent for such substitutions (DMF, DMSO); it should lead to a parent
molecule of tri-FOX
(-C(X)=N-)3 + 3 C(-C#N)(NO2)2(-) --> (-C(-C(-C#N)(NO2)2)=N-)3 + 3 X(-).
X = Cl, Br or I
The resulting 2,4,6-tris(cyanodinitromethyl)-1,3,5-triazine should be hydrolysable into water under its own acidity:
(-C(-C(-C#N)(NO2)2)=N-)3 + 3 H2O --> (-C(-C(-CO-NH2)(NO2)2)=N-)3
(-C(-C(-CO-NH2)(NO2)2)=N-)3 + 3 H2O --> (-C(-C(-CO2-NH4)(NO2)2)=N-)3
(-C(-C(-CO2-NH4)(NO2)2)=N-)3 --> (-C(-C(NH4)(NO2)2)=N-)3 + 3 CO2(g)
The final result would be tri-FOX triammonium salt.
[Edited on 3-5-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
|