UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
"What are you working on?" Thread
On a small chem forum I used to frequent, we had a thread like this and it went on quite pleasantly for some time. No need to post finished preps, or
even particularly interesting preps. Pictures welcome of course. What chem projects are you working on?
A lot of users probably never post because they feel they are doing inconsequential things. Sometimes a writeup comes from a completely unexpected
source since they've been keeping quiet about most of their work. I'd like to see how active our users are on a general basis.
I myself have a bad habit of working on a dozen things at once. I've got "fractionated coconut oil" biodiesel that I intend to vacuum fractionate for
it's methyl octanoate, decanoate, and laurate content. The octanoate is of particular interest, as a bouvealt-blanc reduction will give octyl alcohol.
Octyl acetate is an important fragrance/flavor compound. The biodiesel itself smells from the volatile medium-chain fatty acid methyl esters. While
the parent oil was odorless, it smells like coconut mixed with watermelon jolly ranchers.
I'm also getting back into my synthesis of capsaicin. I have everything to make the necessary vanillylamine.
My dessicator contains caffeine methiodide (1,3,7,9-tetramethylxanthinium iodide). In the presence of base, this can form an N-heterocyclic carbene
and metal complexes. I made it with the hope that it serves as an efficient and nontoxic catalyst for the benzoin condensation
The dessicator also contains alpha-ketoglutaric acid phenylhydrazone. This, subject to Fischer indole synthesis conditions, gives a product that
through decarboxylation furnishes indole-3-acetic acid, a plant auxin.
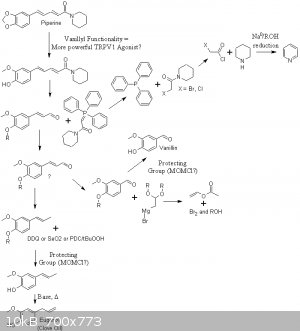
I am also trying to work out a synthesis route for the vanillyl analogue of piperine in hopes that it is a more powerful TRPV1 agonist than the parent
compound. Potential scheme attached.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
At the moment, dry isopropanol. Yesterday I managed to get about a gram and a half of raw clove oil out of 24 grams whole cloves. Time well wasted
LOL
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I currently have two projects: an induction heater (ZVS topology) and a manganese ammonium sulfate cell for the electrolytic oxidation of toluene to
benzaldehyde.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by arkoma  | | At the moment, dry isopropanol. Yesterday I managed to get about a gram and a half of raw clove oil out of 24 grams whole cloves. Time well wasted
LOL |
That's actually not terribad for yield. Did you extract the distillate with DCM or hexane or something?
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Currently I have no lab, but I have several projects planned for the future. This is the biggest one, but I also want to work a far more advance subject - separation of lanthanides using ammonium citrate and
nitrilotriacetic acid (I've read a few things about this) and thermiting/electrolyzing them into the pure metal.
I also might attempt to work out electrolysis of anhydrous metal salts in ionic liquids.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Brain&Force: NO LAB? for why? I am just about 200mi from mine, but it still exists.
last time I was working there( not just cleaning old projects and recrystallizing), was starting a steam generator for plant essence distillation.
there was some work with small scale sulfamic acid electrolysis experiments: 1) stripping PCB boards, 2) silver plating experiments 3) cupronickel
separation of Ni. and finally extracting the UV active plant dye in osage orange wood/bark.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I live on a military base. And I'm holding out for university in ~3 months.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
prof_genius
Hazard to Others
  
Posts: 147
Registered: 15-5-2013
Member Is Offline
Mood: No Mood
|
|
Im on vacation in Australia, I will probably resume work on copper compounds when I get back.
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
gotcha, ok that makes sense. a lot to look forward to soon, nice. for me, it's not too bad while away, with all the interesting things to read up on
here to help or just join in a discussion. its nice to get to experiment, though just reading can be a worthwhile fill in short term.
the ionic liquid electrolysis has caught my attention a few times also. haven't tried it yet.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by UnintentionalChaos  | Quote: Originally posted by arkoma  | | At the moment, dry isopropanol. Yesterday I managed to get about a gram and a half of raw clove oil out of 24 grams whole cloves. Time well wasted
LOL |
That's actually not terribad for yield. Did you extract the distillate with DCM or hexane or something? |
Not yet. Have a tiny bit of diethyl ether saved though........mebbe later this morning--just got up (5:54AM here)
Edit wanna do this procedure, basically
[Edited on 7-15-2014 by arkoma]
Attachment: extraction of eugenol from cloves.pdf (204kB)
This file has been downloaded 8711 times
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I am working on a total synthesis of hydroxylammonium 3-amino-4-nitrofurazan from strictly OTC
chemicals.
So far:
Na2S2O5 + H2SO4 --> SO2
Na2SO3 + KNO2 + SO2 --> Hydroxylamine
NH2OH + Acetone --> acetone oxime (Extraction step)
Acetone oxime + HCl --> NH2OH-HCl
EtOH + Na2Cr2O7 + H2SO4 --> acetaldehyde
Acetaldehyde + HNO3 --> glyoxal
Hydroxylamine HCl + Glyoxal + NaOH -->
Diaminoglyoxime
Diaminoglyoxime + KOH (in 1,2-ethanediol) --> Diaminofurazan
Diaminofurazan + Potassium Peroxymonosulfate --> 3-nitro-4-aminofurazan
3-nitro-4-aminofurazan + NH2OH-HCl (basify to precip.) -->
HAANF
The biggest issue so far has been sourcing glyoxal. I prefer to start with ethanol and oxidize half with cheap HNO3 to minimize the use of expensive
dichromate, rather than go the 1,2-ethanediol/dichromate route.
Alternatively, I would begin with OTC 1,2-ethanediol and dehydrogenate right to glyoxal at 450C over a copper catalyst. I am currently designing an
apparatus for this, since it would mean an unlimited cheap source of formaldehyde, acetaldehyde, and glyoxal. This would also make a total OTC
pentaerythritol synthesis trivial, and I could possibly sell small amounts.
Anyway, the reason I want to try HAANF is because, to my knowledge, it is the most powerful chemical explosive synthesized in gram
amounts with reported sensitivity data. A paper I have shows a VoD of 10,010 m/s @ 1.89 g/ml, with a Pcj of 478 kbar. Compare to HMX at 9059 m/s @
1.92 g/ml and 392 kbar and CL-20 with 9342 m/s @ 2.04 g/ml and 446 kbar. It is also less sensitive than RDX in friction, shock, and static.
Just so I can say that I did it. 
My other pet project is triaminoguanidinium bis(2,2-dinitroethyl)nitramine. I won't get into synth details but it's pretty straightforward. Klapötke
says 10,004 m/s @ 1.99 g/ml and 462 kbar with good sensitivity.
[Edited on 15-7-2014 by Praxichys]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by Praxichys  |
Na2S2O5 + H2SO4 --> SO2
Na2SO3 + KNO2 + SO2 --> Hydroxylamine
NH2OH + Acetone --> acetone oxime (Extraction step)
Acetone oxime + HCl --> NH2OH-HCl
|
Use MEK instead of acetone. The oxime seperates readily as a liquid (in okayish yield) and has reduced volatility. If you haven't run the prep yet,
know that the distillation with HCl has to be agonizingly slow or the oxime itself distills. Oximes are something like 1000 fold more stable to
hydrolysis compared to azines/hydrazones.
Hydroxylammonium chloride forms huge, beautiful crystals though, so the hours of frustration will at least have a redeeming ending.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
@ UC - thanks for the insight.
I planned to L/L extract the acetone oxime but I think you're right about the MEK being the better route. I will salt up the mother liquor to better
facilitate the phase separation.
I imagine the loss of HCl is probably the biggest problem in the distillation, since the BP of everything else is pretty high. I will probably heat
the oxime with water and excess HCl and stir overnight at about 80C, taking excess HCl and the ketone out the top of the column and forming a
concentrated hot solution of hydroxylammonium chloride in the bottom. The endpoint determination remains an issue.
The liquor will be rapidly stirred under aspirator vacuum while cooling to remove excess HCl, then stuck into the fridge for the salt to fall out of
solution. Recrystallize, etc.
Hydroxylammonium sulfate looks attractive as an alernative because I can use much higher temperatures for the hydrolysis, up to the BP of the ketoxime
(something like 152C for MEKO). I just don't know how this is going to interfere with the extraction of diaminoglyoxime since sodium sulfate is much
less soluble then sodium chloride. There is some research yet to do.
I am also excited to see if, in parallel to Axt's metal glyoximates, whether diaminoglyoximates can also be formed. (Carefully...)
[Edited on 15-7-2014 by Praxichys]
|
|
|