enima
Hazard to Self
 
Posts: 98
Registered: 1-12-2004
Member Is Offline
Mood: No Mood
|
|
Synthesis Ideas for 4-bromo,2,5-dimethoxybenzaldehyde.
Hey, its been a nice winter so far, lots of time to think about different ways to synthesize this compound.
Give me your opinion/suggestions on the following methods.
Starting with hydroquinone.
Methylation of hydroquinone
a. Methylation using betaine anhydrous and CaO to produce the dimethyl ether.
*-prefered method as bentaine isn't carcinogenic like tmp/ dms/mei.
b. Methylation with trimethyl phosphate, and k2co3 under a nitrogren atmosphere.
c. Methylation with dimethyl sulfate and K2CO3.
Bromination of 1,4-dimethoxybenzene
a. Bromination using H2O2 / Ammonium Bromide in a GAA solvent.
b. Bromination with Oxone/ NaBr using H2O as the solvent.
Now you have your 1-bromo,2,5-dimethoxybenzene compound. Coverting it to the aldehyde requires a formylation of sorts. Unfortunately the R/T
formylation cannot be used since you are no longer working with a phenol group.
3a. Bromomethylation.
The 1-bromo,2-5-dimethoxybenzene compound is bromomethylated using NaBr, H2SO4 and paraformaldehyde to produce the bromomethyled compound.
From here we can go two ways.
1. SN2 replacement on the bromine with OH, via hydrolysis of the compound in alkaline ph.
----> Oxidation with DMSO to the aldehyde.
2. SN2 replacement with NaNO2 (similar to the synthesis of Nitroethane from bromoethane).
----> Oxidation with NaClO2 (Nef Reaction) to produce the aldehyde.
3b. Formation of the mandelic acid and then oxidation.
React the 1-bromo,2,5-dimethoxybenzene with glyoxylic acid and a strong acid (sulfuric acid) to produce the mandelic acid.
React the mandelic acid with sodium persulfate using a silver nitrate catalyst to oxidize the mandelic acid to the aldehyde.
* The reason I am proposing the dimethoxybenzene route is because hydroquinone is cheap and easily obtained, the R/T formylation of p-methoxyphenol
requires methylation (to produce the 2,5-dimethoxybenzaldehyde) using carcinogenic methylating agents such as dimethyl sulfate and methyl iodide.
Methylation of non-aldehyde phenols can be done using bentaine at temperatures at 180-200C. (These temperatures will destroy the aldehyde group).
-enima
[Edited on 11-1-2005 by enima]
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
the name 1-bromo,2,5-dimethoxybenzaldehyde isnt correct for a start....
do you mean 4-bromo-2,5-dimethoxybenzaldehyde as in the precursor for 2C-B?
Otherwise please revise your name as its incorrect, without knowing what you want to synth we cant help easily.... or even at all...
-rlr
[Edited on 10-1-2005 by runlabrun]
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | | The reason I am proposing the dimethoxybenzene route is because hydroquinone is cheap and easily obtained, the R/T formylation of p-methoxyphenol
requires methylation (to produce the 2,5-dimethoxybenzaldehyde) using carcinogenic methylating agents such as dimethyl sulfate and methyl iodide.
|
Is it because you are so worried about handling carcinogenic alkylation agents that you thought of the bromomethylation step?
[Edited on 11-1-2005 by Sergei_Eisenstein]
|
|
|
enima
Hazard to Self
 
Posts: 98
Registered: 1-12-2004
Member Is Offline
Mood: No Mood
|
|
yes mainly, I'd rather have fun with with a corrosive lachrymator than deal with methylated inactivated dna  . .
The glyoxylic acid method IMO seems most favorable. The product intermediate, the mandelic acid, would be nicer to work with than the substituted
benzyl bromide. The only problem is the glyoxylic acid. (Although it can be synthesized through the reduction of oxalic acid or the oxidation of
glyoxal.)
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Chloromethylation with HCl/formaldehyde produces also the very highly carcinogenic bis-chloromethyl ether (lung cancer), Cl-CH2-O-CH2-Cl, as all
mixtures of formaldehyde and HCl do, so it is expected that H2SO4/NaBr/formaldehyde should produce a similar bromo compound. It's volatility
might be lower but I bet it is even more carcinogenic. It should be possible to perform the reaction relatively safely with precautions, but shit
happens. I think both ethers are decomposed by alkali.
Benzyl bromides react via SN1 but the reaction with NaNO2 is unlikely to give good yields. AgNO2 would be a better pick, like the traditional way of
nitroalkane synthesis.
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | Originally posted by trilobite
Chloromethylation with HCl/formaldehyde produces also the very highly carcinogenic bis-chloromethyl ether (lung cancer), Cl-CH2-O-CH2-Cl, as all
mixtures of formaldehyde and HCl do, so it is expected that H2SO4/NaBr/formaldehyde should produce a similar bromo compound. It's volatility
might be lower but I bet it is even more carcinogenic. It should be possible to perform the reaction relatively safely with precautions, but shit
happens. I think both ethers are decomposed by alkali. |
My point exactly  Always take the necessary precautions when handling benzyl
halogenides. In my opinion, it does not make sense to avoid DMS due to its carcinogenic properties, but yet employ a route using benzyl halogenides
when other, "safer" routes exist. If you take the necessary precautions, handling these substances is not really a problem. It only becomes
really dangerous when you handle them unprepared and uninformed about their possible risks. Not just dangerous for you but also for others. Always take the necessary precautions when handling benzyl
halogenides. In my opinion, it does not make sense to avoid DMS due to its carcinogenic properties, but yet employ a route using benzyl halogenides
when other, "safer" routes exist. If you take the necessary precautions, handling these substances is not really a problem. It only becomes
really dangerous when you handle them unprepared and uninformed about their possible risks. Not just dangerous for you but also for others.
|
|
|
incitatus
Harmless

Posts: 3
Registered: 18-1-2005
Member Is Offline
Mood: No Mood
|
|
aldehyde route
other formylations such as the vilsmeier are viable for methyl ethers and have less risk of carcinogen....so reagent of pocl3 and DMF.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
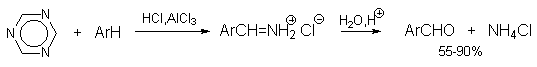
http://www.chem.msu.su/rus/teaching/aromat/part(3.5).html
This seem to bee a very interesting way to formylate, it reminds somewhat of Gatterman. I found no litt. refs in that link. But, the price of the
1,3,5-triazine is outrageous, can someone post preparation?
[Edited on 6-7-2005 by Sandmeyer]
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
One reference for the formylation reaction is Z. Chem., 10(10), 383-384 (1970). It is called the hydrocyanic acidless Gattermann formylation
(blausäurefreie Gattermannsche aldehydsynthese). I have a vague recollection that there might have been another ref too but I couldn't find it.
In this one they perform the reaction on a few aromatic hydrocarbons (indane, fluorene etc)
One reference for the synthesis of triazine is J. Am. Chem. Soc. 81, 1466-1470 (1959):
"Five grams of formamidine hydrochloride was heated gradually to 250°C at 20 mm pressure. Visible decomposition began at a bath temperature of
120°C. Volatile decomposition products were passed into a receiver chilled with Dry Ice. Crystalline sym-triazine which collected weighed 1.2 g.,
m.p. 80-81°C, yield 72%."
They give references for other preparations too, J. Chem. Soc. 1930, 1834; Annalen 287, 337 (1895); Berichte 42, 1915 (1909). I think I copied the
JACS article because it didn't involve hydrogen cyanide. They say that this reaction gives low yields without vacuum.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Synthesis of "4-bromo-2,5-dimethoxybenzaldehyde" from "1-bromo-2,5-dimethoxybenzene"(which is actually called 2-bromo-1,4-dimethoxybenzene) seems like
a waste of good 2-bromo-1,4-dimethoxybenzene to me. There's not much of a point. 2-bromo-1,4-dimethoxybenzene has so many possibilities. Oh well, to
each their own. But thanks for posting! It's always useful to share chemical knowledge.
I'm so cool, I'm supercooled! 
"Imagination is more important than knowledge" ~Einstein
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
A useless post on an 8 year old thread
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Sorry about that. I screwed up and wasn't sure what to post. I recently came across somewhat useful information. I found out that the hyacinth
produces 1,4-Dimethoxybenzene. I was thinking maybe the raw compounds could be separated via hydrodistillation and then the 1,4-Dimethoxybenzene could
be obtained from that. Some people use it for biological research (I think proteomic or something). Science!
I'm so cool, I'm supercooled. 
"Imagination is more important than knowledge" ~Einstein
|
|
|
psychronizer
Harmless

Posts: 6
Registered: 21-3-2013
Member Is Offline
Mood: No Mood
|
|
I'm intrigued...hyacinth makes 1,4 dimethoxybenzene?!?! really? in what quantity? theoretically it would be just perfect for a Gatterman type
formylation, or perhaps a derivative thereof...
|
|
|
Nitrous
Harmless

Posts: 2
Registered: 2-8-2013
Member Is Offline
Mood: No Mood
|
|
Is GAA glacial acetic acid?
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, no good can come of this, but......a similar product might be obtainable commercially.
2,5-Dimethoxy-4-Chloro-Aniline, is available commercially. It isn't a great stretch of the imagination to think that 4-Bromo might be attainable
somewhere.
The Chloro product is used in the production of some Yellow pigments/dyes, and it could be recovered from them.
I'm assuming this Aniline can be diazotized and converted to other functional groups. May or may not be possible.
Of course, if the object is 2CB. Desirable, because it is short acting, the 4-Chloro.....doesn't really cut it.
http://www.chemicalbook.com/ChemicalProductProperty_EN_CB067...
etc.
[Edited on 1-10-2013 by zed]
[Edited on 1-10-2013 by zed]
|
|
|