| Pages:
1
2 |
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemosynthesis  | Your stereo chemistry would be correct, but you placed the bromine and hydroxyl group incorrectly, so your regiochemistry is mistaken.
When performing a Markovnikov addition, the most electronegative group adds to the most substituted or stable carbon. Oxygen is more electronegative
than bromine, so alcohols or ethers form on the most substituted carbon here.
Some confusion may come from acid addition; If it were HBr addition, bromine is more electronegative than hydrogen, so the bromine would be on the
more substituted carbon. If you have elemental bromine in an inert, aprotic solvent, the dihalide is equally electronegative and so it doesn't matter.
Edit: if you don't want to just memorize this, think about the mechanism that is occurring. In organic chemistry, you will see a pattern of
nucleophiles attacking electrophiles. The way we draw electron arrows is supposed to convey this, because your negative charge is what you start the
arrow from.
When you look at your products here, the double bond is a source of electron density, and so if there is a reaction, it will attack the least
electronegative group. In the case of HBr, this is hydrogen. In your example, it is bromine. Now you are stuck with a positive charge in your
resonance structures. Consider where it is most stable. Your remaining nucleophile (-OH) logically attacks this and adds to that carbon.
[Edited on 7-11-2014 by Chemosynthesis] |
I swapped the Br and the OH- onto opposite carbons, but it still came back wrong. Since the regiochemistry was incorrect I tried to rearrange the
molecules but it still came back wrong. Here's my most recent attempt. Like I said, the way the online software is set up I don't know if I'm drawing
the molecule completely wrong or if I've misplaced certain molecules.
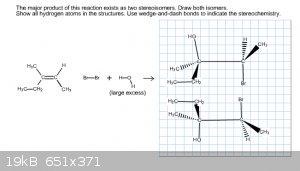
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Never mind, I finally got the answer. Not only were my structures incorrect, but I had the incorrect molecules selected for the dashes and wedges.
It's a relief to finally get this figured out! Now I know what to do for future problems like this!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Thanks for posting the text. Would mind posting the title/author?
Thanks,
CRX
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Okay, this one has me really puzzled. I thought that in order to get from acetylene to trans-2-pentene I needed to first convert the acetylene into
propyne, and then convert that into 1-butyne in order to achieve the trans-2-pentene. But I don't know if I have the mechanisms for each reaction
wrong or if the products for each reaction is wrong. Below is an image of my latest attempt with this problem.
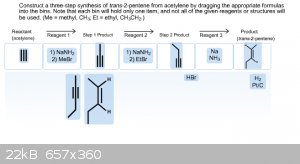
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Your step 2 product is missing the methyl substituent from the previous acetylide substitution. I don't see anything else that looks out of place.
Acetylene to acetylide, substitution. Repeat with different alkyl halide on remaining terminus. Dissolving metal trans reduction pdt.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
According to Markovnikov's rule, the hydrogen should add to the carbon with the most hydrogrens, right? When doing the problem below, this is what I
thought but I'm still getting this wrong. Are the chlorine and the hydrogen supposed to be attached to the same carbon?
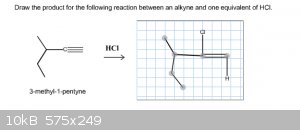
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Look at the mechanism. If you ever have a carbocation intermediate, you have to watch out for methyl or hydride shifts.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
I'm working on some word problems, and the question asks me what what major product forms from the acid catalyzed hydration of 1-phenylcyclohexene. I
drew what I think is the mechanism below, leading to the product 1-phenylcyclohexanol. Am I close?
Update: I just realized that my drawing in the context of this question doesn't make any sense. For the acid catalyzed hydration to occur then the
reagents reacting with the reactant has to be H2SO4 and H2O and not what I listed below. So this drawing is completely wrong anyways.
But would the product of the hydration be similar to the product shown below?
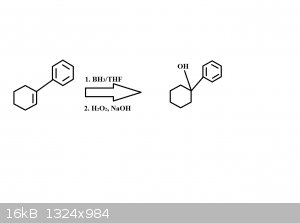
[Edited on 12-11-2014 by Tricia]
[Edited on 12-11-2014 by Tricia]
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
I was able to solve the above question (I needed to add a hydrogen to the ring) but now I have another question. I need to properly convey in my
report as to why the NMR I have reads a syn addition product that follows Anti-Markovnikov's rule. It's a hydroboration reaction involving
1-phenylcyclohexene with an end product of trans-2-cyclohexanol. Our professor went over the NMR in class and indicated that the NMR has six peaks
near 4 ppm, indicating that it was Anti-Markovnikov. There is also a very high peak near 8 ppm, indicating that it's syn addition.
So the issue I'm having is that I need to explain why it's Anti-Markovnikov because of the six peaks and why it's syn addition because of the
high peaks. All I have is my professor's say-so on this, and when I tried to do some research into this topic anything close to what I was looking for
asked me to pay for an answer. I'm not asking for an answer to this, but if somebody could provide me links that can help me with this (and so I can
develop a works cited page other than by what my professor said) I would greatly appreciate it.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
You want to look into peak integration and how the chemically equivalent hydrogens (H-NMR/P-NMR assumed) are indistinguishable.
http://chemwiki.ucdavis.edu/Physical_Chemistry/Spectroscopy/...
http://www.chem.ucla.edu/harding/ec_tutorials/tutorial49.pdf
http://users.wfu.edu/ylwong/chem/nmr/h1/integration.html
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
It's funny how ones that look simple cause me the most trouble. I'm really stuck on sorting these out, and I honestly don't know which is right and
which is wrong. Can someone clue me in if I'm on the right track? Thanks!
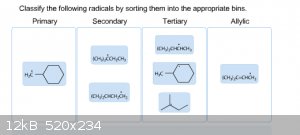
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Try drawing out the condensed structures. The radicals in the primary and allylic bins are correct, but the secondary and tertiary bins are
mismatched. There should be 2 primary radicals, 2 secondary radicals, 2 tertiary radicals, and 1 allylic radical.
[Edited on 11-18-2014 by gdflp]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Count the number of non-hydrogen groups bonded to the radical carbon. If there is one group this = primary, if two groups = secondary, and if three
groups = tertiary. As already said drawing out the radical carbon and the attached groups & hydrogens will make this clear.
Carbon is tetravalent so there will be 3 attached groups/hydrogens and then the lone electron. But you know that already.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
I'm not sure that this question is looking for when it asks for the neutral organic product. I've tried looking this up in my notes, textbook, and
online and I haven't found anything useful. Here's my most recent attempt. Am I close or am I off the mark? Thanks!
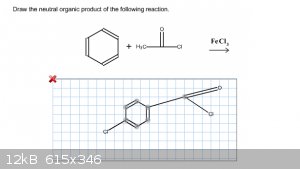
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Remember the Friedel Crafts mechanism; you first have to activate a reagent/catalyst. In this case (acylation), you go through an acylium cation.
There is no chlorination.
The reason it asks for the neutral product is that acylation gives a charged complex of ketone and Lewis acid. This is broken during workup.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
For this problem, the hint stated it was an E2 Elimination reaction. I thought this meant that the Br would be eliminated entirely from the benzene
ring, but that was wrong. Does the methyl attached to the oxygen get eliminated or am I looking at this the wrong way?
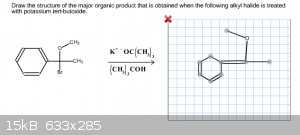
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
I'm out of tries with this problem. Can anyone tell me if I'm close to the correct answer? Thanks!
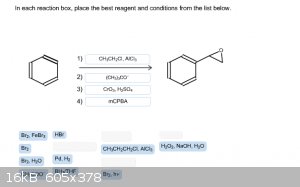
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
Step 2: Br2, hv gives benzylic bromination.
Step 3: KOtBu gives styrene.
Step 4: mCPBA gives styrene oxide.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
KOtBu isn't an available choice.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
KOtBu is not a choice but, (CH3)3CO - is.
Something tells me there's another way with-in the choices but I don't really have time to explore it.
Rather then just giving the answers it might be best to sort of hint at solutions, or explain why a given approach is not appropriate. Then again if
you're 'out of tries' it is what it is.
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
Hence I wrote KOtBu! 
This one's absolutely abysmal: http://www.sciencemadness.org/talk/files.php?pid=367964&...
Perhaps you should re-read the chapter in the book on Friedel Crafts acylation (and alkylation). You obviously haven't understood it at all -- it is
quite central to your further studies that you do.
In this one: http://www.sciencemadness.org/talk/files.php?pid=366544&... you haven't grasped it at all either. There's little point in helping you get the
correct answers because you will struggle with slightly different examples. You've obviously just started with the basics so take this opportunity to
understand it properly; or else you will struggle tremendously later on. Luckily this stuff is the easiest to learn. You will just have to spend an
hour or so reading a chapter in a book. Don't bother trying the questions until you've read over it again, because it would only be guess work.
[Edited on 1-12-2014 by forgottenpassword]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by smaerd  | | Something tells me there's another way with-in the choices but I don't really have time to explore it. |
What about :
1) CH3CH2Cl, AlCl3 to give ethyl benzene
2) Br2, hv to brominate at benzylic position
3) (CH3)3CO- to give styrene
4) Br2, H2O to give halohydrin
5) (CH3)3CO- to give epoxide via intramolecular substitution
Not sure if you're allowed to use the same reagent twice, though. Either way, forgottenpassword's route is the better and more direct one.
|
|
|
| Pages:
1
2 |