| Pages:
1
2
3
4 |
The_Simpsons
Harmless

Posts: 15
Registered: 17-7-2005
Location: Falun, Sweden
Member Is Offline
Mood: No Mood
|
|
The effect of acid on skin
what exactly does acid do to your skin and etc, at the chemical level, (specially strong acids)
sorry for my bad english, english isn't my first language, and im in a kind of hurry
E.b. Chemo: title
[Edited on 13-8-2005 by chemoleo]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I imagine it hydrolyzes proteins. Certainly when it soaks through, the pH imbalance sends your nerves into a frenzy!
Tim
|
|
|
Broken Gears
Hazard to Self
 
Posts: 96
Registered: 7-8-2005
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I have burned my fingers sevel times on acids like: nitric- phosphos- and surfuric acid.
And surfuric acid was by far the most painfull.
The doctor said it was 3. degree burn.
I would love to post some pictures of it, but im not sure i know how.
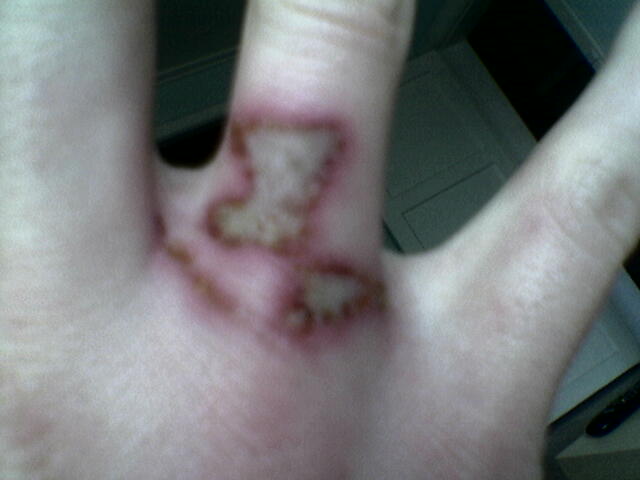
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
Bwaaa, it's only sulfuric acid... Be glad it wasn't chlorosulfonic acid    One
of my friends had a "little accident" while handling chlorosulfonic acid. It burnt through his lab coat (for chemical laboratory work),
longsleeves and gloves. He was quick enough to remove everything immediately, but couldn't prevent that a tiny amount reached his hand. He said
it didn't hurt much, but it was a rather "unusual sight to see your hand smoking". The wound is still visible two years later, and
likely will be for many years to come. One
of my friends had a "little accident" while handling chlorosulfonic acid. It burnt through his lab coat (for chemical laboratory work),
longsleeves and gloves. He was quick enough to remove everything immediately, but couldn't prevent that a tiny amount reached his hand. He said
it didn't hurt much, but it was a rather "unusual sight to see your hand smoking". The wound is still visible two years later, and
likely will be for many years to come.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Of course I'm sure a mixture of fluorosulfonic, hydrofluoric acids and/or arsenic pentafluoride would be quite venomous indeed, but that's
beside the subject of the thread concerning reactions with biological material. 
Hm, forgot to mention, concentrated H2SO4 will chemically dehydrate biology, this would literally be a burn.
Tim
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
i know someone who burned himself with an ionic melt (don't remember which). it looked really bad, the whole hand and a part of the arm had no
skin at all, it took him quite a while in hospital recovering.
a crazy friend tossed some HCl of unknown concentration in my eye unintentionally, it burned like hell,, luckily there was running water 2 seconds
away. i could not see much for a short period, then it was blurry then it recovered. interesting and frightening experience.
[Edited on 13-8-2005 by Sandmeyer]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well think about it- what is the skin made of?
Proteins, Fats, glycosaccharides, water, and pigments.
So... initially your skin is a good protection. Until the outer most layers are soaked enough to allow diffusion to the lower layers.Then you feel the
pain, because nerveendings are killed/stimulated by the different H+ gradients (and celldeath later), etc.
Altogether though, 12Ax is correct of course - hydrolysis of the proteins, of the fats, denaturation of proteins, leading to membrane breakdown, cell
death and so on.
Basically a whole range of things.
As to H2SO4 - I did get 96% onto my hands on a couple of occasions, it started to sting a little that's all. So does conc. hot NaOH solution. But
no lasting effects. I guess I am just a lot tougher than most of you 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Looking for pain and scares ?
Perchloric acid, if you want to know what pain is, then spill this one on your skin. An additional disadvantage with Perchloric acid is, that these
wounds are very slow to heal. I have read stories about chemists that had an accident with Nitrating acid (HNO3 / H2SO4), they pulled there skin off
there hands, only to see there bare bones. An acid which would not be a very likely candidate, based on it's strength, is Hydrofluoric acid.
Again, a very slow healer. Oh, and Sulphuric acid is well known for it's charing properties, so third degree burns are likely to occure. I agree
with Sergei_Eisenstein, Chlorosulfonic acid is a very, very nasty one. Again a very slow healer. When talking about slow healers, I mean wounds that
stay open, there is also a high probability of scars that will mark you till the day you may rest in your coffin. I hope you all may live to a mature
old age, and pass away painless and peacefully though.
[Edited on 14-8-2005 by Lambda]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
| Quote: | | I hope you all may live to a mature old age |
I doubt many of us here will have that pleasure, considering the stuff we play with. Just look at Samosa.
The average chemist doesn't live too long, and we're certainly 'above' average.
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Come on Neutrino !,.... look at the bright side of things. Short and intense or long and boring they say. Forget the "or", and look at it as
long and intense !. Maybe I am just a dreamer, but it shore as hell makes my every day.
[Edited on 14-8-2005 by Lambda]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Hopefully medicine will have advanced by the time all this catches up to me, otherwise I expect to die rather early. Lambda, I have had a rather
severe nitrating acid accident, I was bored and reckless and young so I attempted to nitrate IPA with HNO3 and H2SO4...long story short no personal
protection other than goggles...squirted IPA into mixed acids....mix instantly vapourized and shot straight up at me...left with very painfull burns
on my right hand that were yellow for around a month...I got some strange looks. I also had my shirt melt off my body and pants severly damnaged.
I also had my shirt melt off my body and pants severly damnaged.
[Edited on 14-8-2005 by rogue chemist]
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
I am sorry to hear about this Rogue chemist. I hope your lungs were not damaged ?.
But that's the right spirt, medice will be our saviour, we will all become old ratteling boned men and woman. May all our bones rattle oneday, so
that great, great, grandpa and grandma may pass wisdom on to the spouses.
Yeh, talking about all those torn clothes, you must have looked like the green Hulk in a mood of rage. But if you have received irreversable damage in
the process, then I certainly do not think that this is anything to laugh about. It is not my intention to mock your accident, for this is then a very
seriouse matter. And I certainly hope that things will come right with you.
[Edited on 14-8-2005 by Lambda]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
It was outside, almost no NOx was inhaled, somehow I had the reflexes to move away to avoid acid on my face, other than a few layers of skin and my
pride, I do not think anything else was damaged. Seeing as this was quite some time ago, and I did not die from pulmonary edema, I do not think I
recieved any permanent damage, but thank you for your concern.
It was for sure something to learn from.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
That's the good thing about oxidizers, if they don't kill you immediately, you're basically okay. Except for chromates (cancer) and
permanganates (Parkinsons-esque) anyway.
With all the attention I give lead it's probably going to kill me... it can be chelated, no?
Tim
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
There is a parkinsons like disease from permanganates? I thought the only risk from Mn was infertility in men? Well luckily the parkinsons like
disease only seems to come from excessive inhalation of the dust of Mn compounds...Just don't breathe the fumes when you drip Mn2O7 on
stuff. Not many Mn compounds have high vapour pressures. Not many Mn compounds have high vapour pressures.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yep, but unfortunately a lot of heat can do the same thing... I seem to recall weldors (approx. 0.5-1% Mn in basically all steel, more in special
alloys) and potters (many clay blends and glazes have a lot of MnO2 for black and pink colors) occasionally have trouble with these things.
Apparently there is treatment for Mn, but it takes many uncomfortable weeks to do it or something, and there's probably permanent damage (I
don't know either).
Tim
|
|
|
The_Simpsons
Harmless

Posts: 15
Registered: 17-7-2005
Location: Falun, Sweden
Member Is Offline
Mood: No Mood
|
|
thanks for all replies, now i know
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Wart removal
Being frustrated with the OTC treatments for warts, I take more drastic measures. I use 70% HNO3 and becomes quite painful after 5 seconds. The pain
is simular to 32% HCl on an open cut. Then the skin yellows,, dries, and starts peeling after a couple bathings. I must say I have done this with
90% formic acid and it stung profoundly with a large blister as a result. the blister opened and I had a skin flap, very gross. Formic acid has
vessicant properties that render it undesirable.
Fellow molecular manipulator
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Ouch. Did you get the warts off?
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
These damn warts !
I also once had a wart, and just like you, I got fed up with the normal treatment. When I applied Silver nitrate (hells stone, which decomposes under
the influence of light, and produces Nitric acid in situ) or formula W (I think Salicylic acid) the wart just became bigger or nothing happened. So in
desperation I went into the lab, and got hold of liquid Nitrogen. I poured the liquid Nitrogen out of the main tank into a Dewar vessel, but because
of all the white clouds, it is hard to tell when this vessel is full. A lot of cold gas moved down onto the floor of the 5 * 5 meters filling room,
and it was covered by a 20 - 40 centimeter thick cloud blanket. It's quite scary, for you canot see the floor anymore. When I walked away, I
heard a loud cracking sound. You have quest it allready, I split my rubber shoe sals in half. Both my new shoes where f****d up. So, having been hit
by this rather unpleasant experience, I became cautiouse. I only applied a little Liquide Nitrogen by means of a cotton wool stick onto this wart. And
you know what happend, it didn't go away and instead of being 0.5 cm, it became nearly 2 cm. It somhow had spread out underneath the blister.
This took a wile though to develope. I got so pissed off, that I again applied liquid Nitrogen, only not in a cautiouse manner. I had a hole on the
palm of my hand about 8 mm deap, and 3 cm wide after the blister had been cut open. After having applied a strong antibacterial ointment, the wound
was closed with cloth soaked in the same, with a plaster to hold it in place and keep out dirt. I am now looking at my palm, seeing no scares and not
even the slightest trace of this gaping wound. I realy thought my hand was going to rot off, it looked that bad.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by rogue chemist
I was bored and reckless and young so I attempted to nitrate IPA with HNO3 and H2SO4...long story short no personal protection other than
goggles...squirted IPA into mixed acids....mix instantly vapourized and shot straight up at me...left with very painfull burns on my right hand that
were yellow for around a month...I got some strange looks.
|
I thought I would mention that IPA added to nitrating mixtures also produces tetranitromethane. TeNMe is an extremely potent carcinogen...
Check US patent 4122124. It mentions near the end (on the left side of the second page of text) that tetranitromethane is formed if sulfuric acid is
present in the nitric acid.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
chemoleo:
"...As to H2SO4 - I did get 96% onto my hands on a couple of occasions, it started to sting a little that's all. So does conc. hot NaOH
solution. But no lasting effects. I guess I am just a lot tougher than most of you..."
Translation: your skin will make better leather than ours. 
chloric1 and Lambda, you guys are too harsh on your verruca. Vitamin C works. Really.  (I meant applying a paste of crushed tablets in water, not taking it in internally) (I meant applying a paste of crushed tablets in water, not taking it in internally)
Speaking of acids on skin, CrO<sub>3</sub>/H<sub>2</sub>SO<sub>4</sub> isn't a pretty sight to behold on
otherwise flawless skin.  It healed after around 5 months with extensive scarring. It healed after around 5 months with extensive scarring.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
From personal experience I can say I'd rather have sulfuric acid spilled on me than hot elemental bromine. That takes months to heal. Fuming
nitric is pretty bad too, stains your skin mustard yellow.
Thankfully I've never spilled perchloric acid on me (70% is all that I've ever used). I've also heard that concentrated formic acid is
really painful while conc. HF is very likely to kill you by poisoning (small F ion really gets into your system). IIRC, hydrazine is another bad one
to spill on yourself.
Peroxysulfuric/Caro's acid, that isn't nice at all, extremely corrosive to organic matter (literally as fast as the Hollywood rendition of
it eating through flesh). Just goes to show that being a chemist has its dangers :p! lol anyone else here notice that their sense of smell is
deteriorating? 
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Actually, mine has been improving since I stopped playing with chlorine. 
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by Fleaker
anyone else here notice that their sense of smell is deteriorating? 
|
Definatly
|
|
|
| Pages:
1
2
3
4 |