| Pages:
1
..
7
8
9
10
11
..
33 |
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I posted it in the azides thread back in 2011 as well.
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
Good article. I will remove the one I just posted as we seem to have an excess.
Ok, what you say is true. I am aware that stab sensitivity is a specific type of sensitivity, but I thought it might be an indicator of other
sensitivity increases as well.
Just trying to rationalize really why I haven't heard much about DDNP-Azide mixtures. From the little bit of testing I have done, even very small
amounts of lead azide, for instance, make DDNP perform very well even with no reinforcing cap. I guess the military is very particular, but I still
keep wondering what is the thing I am missing here. It probably is just that storage stability is not quite up to military standards like you suggest.
There is a little information on page seven in the following document regarding health and environmental aspects of DDNP production and use. Some
other interesting items in that paper as well regarding other primary explosives.
Attachment: Environmentally Friendly Energetic Materials for Initation Devices.pdf (1.3MB)
This file has been downloaded 1507 times
[Edited on 16-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
DDNP has elevated temperature related storage issues that are not bad enough to cause its rejection for usually encountered civilian uses but
eliminates DDNP or tends to eliminate it as less desirable for military uses where better tolerance of environmental extremes during very long term
storage is what is wanted.
If the military has an ammo dump sitting in the Sahara desert sun for 10 years, they don't want any of the ordnance to be degraded in storage. The
stability of DDNP is good enough for it being practical for many less rigorous storage requirement civilian uses where its economy and performance are
sufficient. For more demanding military applications where the economy is not a concern there are better materials as choices.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2g of Picric Acid Initiated with 0.3g of DDNP & 0.05g of Lead Azide (Unreinforced Configuration)
This test was the same as the last test, where the lead azide was simply pressed on top of the DDNP, except for using 0.3g of DDNP instead of 0.4g and
using 0.05g of lead azide instead of 0.1g. The results were surprisingly better despite using less primary explosives, which after a little head
scratching was attributed to the ambient temperature being in the high twenties for this test, versus the low teens for the last couple of tests.
Higher temperatures can make explosives more sensitive to initiation and perform better as well.
Look at that fragmentation pattern. Glad I was behind a large tree a safe distance away.
The holes on the right, in the witness plate shots, are from this test.
    
A test was also conducted, using the same quantities, except that silver acetylide double salts was put in place of the lead azide. There was no
detonation in this case, only a very yellow witness plate. The silver acetylide-silver nitrate was made about four years ago, but it still seemed to
snap quite well when lit, although much weaker than the azides for a similar quantity.
  
[Edited on 16-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
If you make YT video-DDNP review, it would be awesome. Does it DDT in a straw, if not does it DDT in 5mm Al tube with open ends. How does it stand
after storing at elevated temperatures for a while? Can it be mixed with Al or KClO3?
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the test with the silver acetylide double salts Brand. I’ve suspected that being a weak primary the DS may fail to kick the DDNP and now
this is confirmed.
Nice tests mate. Keep the good work!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The temperature stability data and the KClO3 information is already available I think in references posted earlier in the thread. What would be the
purpose of mixing with Al is mysterious.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Jimbo Jones  | Thanks for the test with the silver acetylide double salts Brand. I’ve suspected that being a weak primary the DS may fail to kick the DDNP and now
this is confirmed.
Nice tests mate. Keep the good work! |
The silver acetylide nitrato complexe was old as mentioned and each initiator primary has a specific weight toi acheive détonation of a given
explosive...not all will do at a few mg like azides do...but maybe 50mg will !
[Edited on 17-5-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | | The temperature stability data and the KClO3 information is already available I think in references posted earlier in the thread. What would be the
purpose of mixing with Al is mysterious. |
Adding Al will almost always produce more energetic mixture then adding oxidizer. Unless maybe the the oxidizer is reactive nitrogen or something.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
The silver acetylide double salt is one of the primaries that will detonate without confinement even in microscopic amounts. It is rather weak as
primary though. I know that someone had problems setting off ETN with it. It was also outperformed in terms of brisance by nano-Armstrong's mixture.
Ral, while aluminium powder usually makes the material stronger, it will also lower the brisance - not exactly what's needed for a primary.
[Edited on 17-5-2014 by Dornier 335A]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dornier 335A  | The silver acetylide double salt is one of the primaries that will detonate without confinement even in microscopic amounts. It is rather weak as
primary though. I know that someone had problems setting off ETN with it. It was also outperformed in terms of brisance by nano-Armstrong's mixture.
Ral, while aluminium powder usually makes the material stronger, it will also lower the brisance - not exactly what's needed for a primary.
[Edited on 17-5-2014 by Dornier 335A] |
Still better then adding chlorate. Also what do you think will be better for setting off anfo, 10g DDNP or 10g DDNP+2g german dark?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Just did another test to confirm the last test results. I carefully weighed out 2.0g of picric acid, 0.30g of DDNP and 0.05g of lead azide. I took
pictures, but since the result was so similar to the last test, I see little reason to post them. If they are requested I can of course post them. The
temperature today is once again in the high twenties.
Important Point - DDNP pressed to higher density for this test and last
For this test and the last I pressed the picric acid very hard with the lever press, as always, but I also started pressing the DDNP just as hard
since it was felt that higher density DDNP would perform better, in this case, since it was being initiated by lead azide and didn't need to make DDT
on its own. Previously the DDNP was normally hand pressed. I should have mentioned this earlier, but it slipped my mind despite being the most
important point probably.
From what I have read, adding potassium chlorate up to about 25% total weight does very little to change the performance from what it would be for
pure DDNP (in most cases). It is done for the sake of economics, since potassium chlorate is much cheaper than DDNP.
[Edited on 17-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Ral please provide any references for what you are saying about Al addition to a primary. Al could increase secondary blast effect like a thermobaric
effect with air or water as oxidizer for the unburned vapors from a large explosive, but that effect wouldn't likely appear in a small charge like for
a detonator. There are tests that show oxidizer addition can increase the power output. Al can be used as a graining additive to improve handling
and pressing and storage, but it actually is a diluent for the explosive effect of a small charge.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I'm feeling adding KCLO3 in MF is more to reduce cost, generate hot KCl product and reasons like that. Adding Al would make the mixture horribly
sensitve, but I suspect it will be better, volume basis then adding KClO3. Pb3O4 and flashes can set off anfo and are much slower then for example
DDNP/fine sand-80/20.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Your feelings, suspicions, and information is not entirely correct. There can be an economy improvement for adding an oxidizer because the performance
increase of the mixture allows using less weight of the active component and even less total weight for the mixture as compared to the single
component. You need to look at the data.
[Edited on 17-5-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Table taken from the report, "DDNP a Detonating Explosive", which is widely available on this forum and on the web. I have seen other references as
well. I know the sand test doesn't tell the whole story, but it is a strong indicator.

[Edited on 17-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | Your feelings, suspicions, and information is not entirely correct. There can be an economy improvement for adding an oxidizer because the performance
increase of the mixture allows using less weight of the active component and even less total weight for the mixture as compared to the single
component. You need to look at the data.
[Edited on 17-5-2014 by Rosco Bodine] |
Yet I still think it can be mathematically proven MF/Al 80/20 is superior in every way then MF/KClO3 70/30 based on volume. I'm not convinced either
of the mixtures will be better primary then pure MF volume based above few milliliters of volume.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Aluminum is a fuel and it needs oxygen to release its energy by combustion. I can see the sense of small amounts of aluminum being used in certain
primary mixtures, but not 20%. Potassium chlorate can be made to detonate, especially when in combination with other explosives, and it provides heap
loads of oxygen as well. The most effective blasting caps use the most brisant explosives. Increasing the proportion of fuel, or oxidizer, will
eventually result in primary explosive dilution to the point that brisance and initiating ability suffers. With primary explosives we are limited to a
certain extent because it is difficult to find an explosive with the right amount and type(s) of sensitivity and the highest amount of brisance
combined in the same material. This is one of the main reasons why we usually couple primary explosives with the most brisant secondaries as base
charges.
I doubt that it can be proven mathematically that what you are saying is true. Math is a great tool, but it is still just a tool. Some things, and
this is one of them, have to be actually tried in real life testing. Don't get me wrong mathematical principles can often get you very close to
reality, but real life testing almost always trumps anything done on the black board IMHO.
[Edited on 17-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
MF is not even chemically compatible with Al so it is really beside the point for the particular combination. In ASA the Al is used as a release
agent for the press pins that tend to get caked with Pb styphnate probaly as a result of some of the gelatin or PVA or dextrin traces in the Pb Azide,
and possibly other binders in the mixture. The Al used at 3% of the composition is a flake variety which tends to leave a physical barrier lamination
surface and layers like shingles on a roof or plywood which seals a compressed pellet of the ASA from anything beyond a superficial effect of
atmospheric degradation or slow decompositon of the Pb Azide. ASA is 68% Pb Azide 29% Pb Styphnate 3% Al
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I'd have to correct myself, 20% by weight(of that heavy MF) will take a lot of the volume. I don't understand the purpose of balancing oxygens here? I
care only about velocity of the composition, energy density, brisance, gas volume etc. It won't take much Al to exceed the energy density of
MF/KClO3. I'm only not sure if the DDT of the mixture will be right.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Aluminum mixed with a high explosive tends to release most of its energy after the high explosive shock wave passes through, if my understanding is
correct. It does release tremendous amounts of energy, but the way it releases its energy is not normally very useful for initiating other explosives.
The military has put huge amounts of energy and expense into getting aluminum powder into forms that will release its energy in a more desirable way
explosively (finer particles, shape, etc). What is desired for initiation is a very high velocity "snap", that will disrupt and break apart the
molecules of the explosive to be initiated. It usually has much less to do with total energy release than brisance. I think it was you earlier in this
thread that pointed out that lead azide has relatively low energy, and yet it is still one of the very best initiators. DDNP has extremely high
energy, but its initiating ability is normally less than lead azide. For an extreme example, flash powder with lots of aluminum powder, releases huge
amounts of energy, but it is a horrible initiator of detonation.
Heat of explosion of lead azide : 391 calories per gram (Explosives, Meyer)
Heat of explosion of DDNP : 820 calories per gram (Military Explosives)
Heat of explosion of perchlorate-Al flash powder : ~2200 calories per gram (Sam Barros)- I didn't verify by calculation
Obviously the most important difference between these explosives is not total energy release.
DDNP oxygen balance : -60.9% (also something to consider, from Meyer)
[Edited on 18-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | DDNP has extremely high energy, but its initiating ability is normally less than lead azide.
[Edited on 18-5-2014 by Hennig Brand] |
There's nothing extreme in the DDNP. NG/PETN jelly is what can be called with such superlatives. Per unit weight the azide is excelent btw. I don't
ague that adding Al will improve MF. I argue that adding a little Al will be better(in performance) then adding KClO3. And when it comes to anfo-if it
can be set off with a flash, I'd trust it can be set off with a moderately aluminized explosive. Btw these "moderate amounts" of aluminium can make
acetone peroxide more energetic then RDX.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I'm calling B.S. on this OT departure into "feelings, suspicions, and speculations" that aren't backed by data, while good data says the opposite.
Let's see some data to back up what you are continuing to argue, now even venturing into the tall grass of speculative Al enhanced peroxide you
speculate is "more energetic" than RDX. "More energetic" would be a pretty abstract argument for that scenario. And it certainly would
not be more brisant. Diesel fuel is "more energetic" than aviation gasoline, but the ignited fumes of the avgas will do a much better
job of blowing a hangar into more numerous smaller pieces. Blast effect that gains secondary energy like an afterblast enhanced effect thermobaric
detonation is a different matter entirely for large secondary explosive detonations and does not apply in detonator scale devices where the minature
firing train is an initiation sequence, and what Hennig says is right on point, while your arguments are not correct. Even for a pure substance like
Pb Azide there is a velocity penalty and decrease of initiating ability for any diluent whatever, like a trace percentage of dextrin or PVA or
gelatin, but can be regained by addition of an active like KClO3 which does itself detonate under the condition of the firing train, while inactives
like Al or other nonexplosive "fuels" do not detonate. Mixing an active material like ETN or MHN with DDNP can increase the output, or mixing another
more unequivocal primary explosive active like Pb Azide or Ag Azide likewise would be expected to enhance the performance, but just as likely it would
be expected that adding any inert non-explosive fuel would tend to quench the detonation wave for what is already an oxygen negative balanced
composition, where no thermobaric secondary effect is going to be of any use in a scheme where the brisance of the initiator is what is mainly the
insult to the base charge that will provoke its detonation. There is a possibility that some metal ions can be catalytic in lowering the threshhold
for the brisance that is required to initiate a particular base charge, but usually it is a heavy metal ion like lead or silver or copper that can
cause that peculiar catalytic sensitivity, and adding high fuel value nonexplosive metals or other fuels to any oxygen deficient explosive is only
going to generally slow it down and that effect is worse in a small scale device like a detonator where the base charge has already been disrupted by
the initial detonation wave before there is any chance for a secondary thermobaric blast effect to arrive. A detonator scale device is not going to
gain any fuel value afterblast bubble energy like from the detonation of a ton of torpex in seawater, because the base charge is not still sitting
there like would be the hull of a ship waiting on the bubble energy of the Al with vaporized seawater. The base charge in a detonator is already long
gone before the Al has any chance to ignite with any environmental supplied oxidizer.
The environment inside a detonator is millimeter scale, not meters of reaction zone where another bubble energy effect could occur using an
environmental oxidizer as a supplement.
As for the use of pyrotechnic mixtures of "flash" or thermite compositions which can detonate, the scale and mechanism of detonation is different and
the velocity is slower and the scale of the devices is much larger to a point that although it is possible to use such devices as detonators, it is
not generally practical. There are a lot of better schemes that can be implemented as a crude improvisation to use as a substitute detonator.
Electrical schemes are the most efficient alternatives to pyrotechnics. That is a different art and there isn't much or any overlap with what is being
done using DDNP.
[Edited on 18-5-2014 by Rosco Bodine]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Thank you for spending so much time on my useless off topic discussion. If you ask me, a good detonator would be fine RDX in aluminium case from
arrow, initiated by azide with reinforcing cap that is protected from the RDX storage decomposition products. But sill,
mixture 1: 70/30 MF/KClO3
mixture 2: 88/12 MF/Al
Do you still think 2ml volume of mixture 1 will make a stronger cap in some way? KClO3 detonates a little better then rocks or something. Cheditte is
a little better then MF as energy density. It's proven the KClO3 significantly reduces the velocity in that mixture. The books talk the lower speed is
only partially compensated by the "hotter flamefront". The density and the small amount of the Al shouldn't make such big impact on the det vel, but
still give a lot of heat(more I think).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Mixture 2 is what, aluminum amalgam and unreacted MF plus maybe aluminum cyanate or other predicted possible corrosion byproducts.......so why don't
you stop beating this dead horse, please.
Yes, mixture 1 is a valid detonating mixture and mixture 2 is not a valid detonating mixture but is a chemical reaction mixture. That ends the matter
right there. You are talking about secondary mixtures that are not within the scope of the conditions presented and you don't seem to get it that the
scale down does not apply. Your arguments are not applicable to detonator scale devices and irrelevant to this thread which you are trashing.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Friction Sensitivity of DDNP
Well I didn't do my own friction testing, but I found a very good report done by the good people at the Institute of Energetic Materials in the Czech
Republic. The journal article is titled, "Sensitivity to friction for primary explosives", by Matyas, et al. I have made jpgs of the three key tables
and the conclusions.
Some very interesting findings in my opinion. DDNP truly is very insensitive for a primary explosive. The results for lead azide and the peroxides
were also very interesting.
Sciencemadness was also in the list of references again, though I think we really should be higher up on the list. 
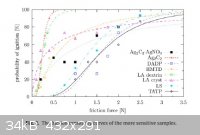   
BTW, reference [6] in the conclusions is Meyer 5th edition.
[Edited on 19-5-2014 by Hennig Brand]
[Edited on 22-5-2014 by Bert]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
..
7
8
9
10
11
..
33 |