guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Decomposition of ammonium salts
Is there a way to determine the decomposition products of ammonium salts? They all seem to decompose to different products.
3(NH4)2SO4 --> 4NH3 +N2 + 6H2O + 3SO2
and
CH3COONH4 --> CH3CONH2 + H2O
and
NH4(NH2SO3) --> N2O + ???
and
(NH4)3PO4 ----> ????
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
In some cases, the hot ammonia/ammonium group has enough reducing power to change the anion. Ammonium dichromate is a good example: it burns with
itself because the chromate is easily reduced while the ammonium is (relatively) easily oxidized.
In other cases, the anion cannot be reduced much (as in the sulfate case), or at all (NH4Cl <--> NH3 + HCl).
Tim
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I'm no expert on this, but I will give you the general trends I've seen.
If the anion is a stable inorganic, it will generally decompose like this at relatively low temperatures, around several hundred *C:
NH<sub>4</sub>X --> NH<sub>3</sub> + HX
(NH<sub>4</sub> <sub>2</sub>X -->
NH<sub>3</sub> + NH<sub>4</sub>HX <sub>2</sub>X -->
NH<sub>3</sub> + NH<sub>4</sub>HX
If the anion is a strong oxidizer, it will form nitrogen and water at these temperatures, or (in the case of
NH<sub>4</sub>NO<sub>3</sub> at lower temperatures), N<sub>2</sub>O.
If the anion is a carboxylate, you should get an amide, e.g. ammonium formate to urea:
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by neutrino
If the anion is a carboxylate, you should get an amide, e.g. ammonium formate to urea: |
Ammonium formate decomposes to FORMAMIDE and water.
I am notice a pattern for carboxylic anions. The oxygen between the carbon and hydrogen (from ammonia) are removed to form water, leaving behind the
amide. The question is, why are those specific atoms being removed? See attachement.
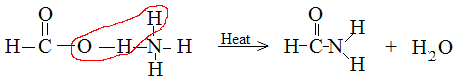
|
|
|
Dr. Beaker
Hazard to Others
  
Posts: 132
Registered: 9-9-2005
Location: between the med red and dead
Member Is Offline
Mood: Shaken, not stirred
|
|
I believe you first get protonation of the carboxilate by the ammonium and then condensation of ammonia thus formed and the acid to the amide (see the
reaction mechanism in any organic texbook). by the way, the 1st synthesis of natural product was the conversion of ammonium cyanate to urea by Wohler.
and a little bit off topic - if someone has a suggestion for a mechanism to the thermolysis of Ca(OAc)2 to CaCO3 and acetone (one can get various
ketones this way by using various carboxylates) I'll love to hear it
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Oh I got it now. First the formate is protonated (forming the formic acid), giving off NH3. Then a nucleophillic reaction occurs forming the amide
and giving off H2O. Thanks, I am new to organic chemistry. I just did some research, so now I get it.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Decomposition of NH4+ salts with Metal Hydroxides
I have read that ammonium salts when heated with a metal hydroxide or oxide or carbonate, it will form ammonia and the metal salt. I tried this
method with Fe2O3 and (NH4)2SO4 but I did not make any Fe2(SO4)3. I heated it to the point where it was forming water. When I tried to dissolve the
product, I didn't dissolve at all.
Does anyone have a detailed description on how to prepare these metal salts from ammonium salts?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Traditionally this is done in aqueous solution so the reactants mix well enough to react at a noticeable speed. Just mixing the solids won't cause a
reaction because they aren't mixed intimately enough. The high lattice energy of these oxides won't help, either.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
OK,
I mixed them up in water first, then I evaporated it so they are very thourougly mixed. So the problem is that the high lattace energy of Fe2O3.
What are some better oxides? CuO, PbO?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Try CaO, adding a drop of water to get the reaction going helps as well.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
If CaO works then PbO should work since their lattic energies are nearly the same (CaO = 3414 kJ/mol , PbO = 3520 kJ/mol). And I can prepare PbO by
heating lead fillings in air. What temperatre should I heat this to?
The procedure is (i think):
Mix PbO and (NH4)2SO4, then add water to mix throughly. Then heat to ??? until ammonia smell is gone.
PbO + (NH4)2SO4 ----Heat---> PbSO4 + 2NH3 + H2O
[Edited on 12/10/2005 by guy]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
To correct myself, I was thinking more of the melting points (which are a function of lattice enregy), as I assumed that you were trying to melt the
first batch. In aqueous solutions, the solubility is more important.
|
|
|
Dr. Beaker
Hazard to Others
  
Posts: 132
Registered: 9-9-2005
Location: between the med red and dead
Member Is Offline
Mood: Shaken, not stirred
|
|
I believe it's a question of basisity of the oxide anion in PbO vs. CaO
lattice energy is composed of electrostatic atraction (which depends on size, charge, and ionic radius) and of covalent bonding in the latice.
therefore, you can have 2 compounds with similar lattic energies but with differend basisity of O2-.
you probably know that the much larger post-transition metals like Pb+2 has a lesser electrostatic atraction power then column II metals and therefore
its oxide is amphoteric while CaO is basic.
|
|
|
Nitro-esteban
Harmless

Posts: 39
Registered: 10-4-2013
Location: Fifth dimension
Member Is Offline
Mood: inert
|
|
Is the decomposition of ammonium formate endothermic?
|
|
|