| Pages:
1
..
8
9
10
11
12
..
48 |
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I broke out the LD plating gear today.. Started with approx 300g/l Pb(NO3)2, one liter cell (tall form beaker) on a stir plate at 55C, about 3g/l
Cu(NO3)2 and with residual NaF from the previous experiments. Plated at ~30ma/cm^2. Stirred, and there was still a little silica dust in solution. For
cathodes, I took pieces of PCB (1/64, single sided) and ran brass threaded rod through two places at the top for the cathode assembly. Washed the MMO
anode with hypochlorite/NaOH/percarbonate solution (aka diversol beer equipment cleanser).
Plating setup on my super messy bench
http://pyrobin.com/files/sdc10030.jpg
MMO compared to PbO2-MMO
http://pyrobin.com/files/sdc10033.jpg
Closeup
http://pyrobin.com/files/sdc10036.jpg
Edit: So, is pH control required with perchlorate cells? I just so happen to have almost 3lbs of commercial K chlorate and may give converting that a
try with this LD anode.
Another edit:
38.5g of PbO2 deposited, 1.75mm anode thickness, 1.21mm original thickness. Coating appears adherent, a little fingernail probing indicates it's not
going to flake off the MMO as easily as the Ti strap that got coated. Coating has a nice uniform texture, some pitting/bubbling but we expected that.
The edges of the diamonds seem to have more pitting than the faces, not sure why on this. The bubbling is something that I believe is caused by low
cell pH. I didn't add PbO at any point and it finished <1 again. Added 40g of PbO to the cell, not all of which dissolved, probably right at
25-27g, just enough to replace what plated out.
[Edited on 11-11-2008 by tentacles]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
I'm picturing a giant vat of saturated sodium chlorate + byproducts... how do they extract the sodium chlorate from the liquor, and importantly, how
do they clean it up?
After adding the KCl (hot, saturated solution, I assume) the KCl falls out. What remains in the solution is a mix of sodium, any excess potassium,
and all the chlorinated ionic species, a real hash. Does this liquor then get recharged with chloride, and resused, despite the possibility of
potassium in the mix?
My own lack of understanding of the metathesis process, and the ease and cleanliness, is why I use potassium chloride at the beginning, but it has
huge drawbacks that I need to overcome. Frankly I do not thing a pumping/recirculating system is possible using potassium chloride... it MUST be
sodium. Thoughts?
This 25 liter cell has had 50 amps through it since Saturday. Chloride levels started at 125 g/l and are now down to 95 g/l, for a yield (so far) of
30 * 25 = 750 grams of chloride converted, meaning there is 2,627 grams of potassium chlorate in there. I'll probably run it to 60 grams per liter,
so another day or two at least. |
If you want to use the K Chlorate for pyro stuff where colour (when burning) is important then stick with your K salts only set up. It is very
difficult to get rid off all Na contamination. Just a little Na will pollute with Na orange colour.
Industry uses cyrstallizers. They are a subject in themselves.
See this board for some stuff on Ammonium Perchlorate from Sodium Perchlorate and it will give you an idea of how they do it. Ammonium Perchlorate is
made in high tonnage quantities.
Don't know of any plant that makes K Chlorate directly from Na Chlorate (solution) coming directly from Na Chlorate cell.
There is some (possibly useful possibly not) info here if you like to go library digging:
_______________
R. Bauer, 'Potassium Chlorate Manufacture',
paper
presented at the Electrochemical Society Meeting,
Boston, May 1968.
________________
Ammonium Perchlorate manufacture:
http://www.sciencemadness.org/talk/viewthread.php?tid=9425&a...
see posts of 6-11-07
If you have a solution of Sodium Chlorate and you want to convert to K Chlorate. Boil off some water untill a cooled sample starts to give a ppt. (of
Sodium Salts).
Stop boiling off water and add some water so that you get no ppt on cooling.
Cool the whole lot of Na solution and start adding cold KCl saturated solution untill you are getting no more ppt. You can add some, let ppt settle,
add more etc so that you can see when ppt has stopped.
Filter and use the mother liquor in your cell.
Thats really the best you can do in a batch system.
If K Chlorate coming out of solution and plugging of pipes/pumps is a problem, use less saturated solutions in your cell.
You should look at the part of your system where you extract the product as seperate from the manufacturing end. If you keep the manufacturing end
hot, then liquid going into a cold (crystallization stage) should give you product without any problems.
You need more chambers that two IMHO. An electrode chamber,
a Chemical Chlorate formation chamber (hot), then the crystallizer (cold). You are going to have to recharge with more KCl. I suggest another chamber
or use the Electrode chamber. No Chlorate (or very little) will be formed in the elctrode chamber, all or most will form in the hot CCF chamber and
hopefully stay in solution untill it reaches your crystallizer.
If you go over to Na and keep adding NaCl to the manufacturing end untill there is enough Na Chlorate in the system for it to start to crystallize
when liquid is cooled (as you are going to HAVE to do if you are wanting a system operating like you desired at the very start), then pipes plugging
up etc will still be a problem if you have not got enough chambers and temperatures to suit.
Most all of the diagrams of cell set ups (patents, articles etc) that you have seen have not been showing you the crystallizer. Just the Electrode
chamber and large Chemical Chlorate formation chamber (or whatever you like to call it).
There is absolutely nothing wrong with the set up you have. Single chamber.
If you like you could place a cloth in it (or perhaps plastic filter material) so that all you have to do is lift it out and give it a squeeze to get
close-to-usable product.
Rince once or twice by 'possing' the cloth + Chlorate in cold water.
Dissolve the solid in clean boiling water, boil some more to get rid of hypochlorites, and recrystallize for near perfect product.
EDIT:
Hello Tentacles,
It is great to see ye old Lead Dioxide coating parafanellia being wheeled out and pressed into action. New ground being broken eh!
The photO's you uploaded seem to be huge as I unable to download (slow connection).
There is a Diamond Shamrock Patent on coating MMO with LD, the only one I know off. Iit is attached.
Hope it is a success. Would suggest a much heavier coat but perhaps you are just seen how it goes.
Careful you magnetic stirres does not make a hole in the bottom of container (or is it glass).
Dann2
[Edited on 12-11-2008 by dann2]
Attachment: United States Patent 4444642.mht (74kB)
This file has been downloaded 1138 times
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
dann: It finally got cold here, and it's rather unpleasant to be outside.. most of the time it's windy + subzero.
Mostly this coating session is just to test the process, if it works then we'll see about thicker, chunkier coatings - if needed. Your test went from
chloride to perc, so the wear was understandably very high.
Have you seen Swede's blog posting on chlorate testing? He's refined the NPAA method considerably, to say the least.
Noone wants to comment on maintaining pH in a perchlorate cell? I can't imagine that no chlorine evolves, but if we add HCl, you're just bringing
chloride into the solution..
[Edited on 11-11-2008 by tentacles]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
The once I ran a Perchlorate cell with pH monitoring (with LD Anode), the pH was inclined to go low so I added NaOH.
I think I read somewhere that Perchloric acid is used in industry if pH goes high??
(Perchloric acid not available in the hardware stores around here!)
It is not too hard to make Perchloric acid from Na Perk. + HCl.
You would only need small amounts.
I know little or nothing about chemistry of Perchlorate cells. Mixing of Anode and Cathode products is not required AFAIK.
The pH control is more to do with anode wear? or process equipment/materials of construction corrosion concerns? I don't think pH effects CE. Many
articles state that CE is independent of pH.
Dann2
[Edited on 12-11-2008 by dann2]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by tentacles
Noone wants to comment on maintaining pH in a perchlorate cell? I can't imagine that no chlorine evolves, but if we add HCl, you're just bringing
chloride into the solution.. |
We seem to be going around in circles again ....... !
pH is 6.6 to 6.8 in commercial operations, mainly for current efficiency reasons.
See my post on page 5 of this thread;
http://www.sciencemadness.org/talk/viewthread.php?tid=5050&a...
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Xenoid: I must not have caught that in your posting, that wasn't even very long ago... So then, it's ideal for us - just dump current in, maybe even
add a touch of KOH to ensure a high pH. The LD plating isn't all that difficult to achieve, but the longer it lasts the better, certainly.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by tentacles
maybe even add a touch of KOH to ensure a high pH. |
Well, no, I wouldn't do that.
Remember MMO coatings do not like high pH, if you have pinholes etc. in your LDO coating you may have problems with the MMO coating degrading beneath
the LDO and possibly causing it to flake off. What I meant was that the high pH of an uncontrolled perchlorate cell shouldn't be to deleterious for
the LDO. Anyway, go ahead and try it, I'm interested in seeing what happens. I might even try plating an Mn or Co oxide treated Ti anode. 
Edit:
Tentacles - how did you estimate the surface area of that Ti mesh to come up with your 30mA/cm^2 plating current - or is that based on just the
overall size of the electrode?
[Edited on 11-11-2008 by Xenoid]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Tentacles, that LD plate job looks nice! And I thought MY bench was messy!  I noticed you used cathodes to provide coverage for both sides... would a copper container acting as a cathode make a good plating cell?
I noticed you used cathodes to provide coverage for both sides... would a copper container acting as a cathode make a good plating cell?
The MMO that Tentacles plated is a "special" MMO. Long story, short version... I started my blog on the APC forum and was contacted by a nice
gentleman in Australia, an amateur Pyro, but a career pool chlorinator cell expert. One of the problems that they were having with pool MMO was idiot
end users who turn their systems on full with no salt, don't clean prefilters, essentially create an environment that is not good for an MMO mesh
anode. I suspect the industry as a whole, early on, were having bad warantee and longevity issues.
From my own research on the chlorate industry, vs. pool chlorination, the anodes made for chlorate production have been perfected for many years. The
users run the anodes in strictly controlled conditions. In contrast, the pool chlorinator research continued, the challenge being durability, bipolar
operation, and the goal was an MMO coating that simply will not fail, under any reasonable circumstance.
The MMO in question was designed for pools. My friend in Australia has attempted to wreck this material with some extreme experiments; boiling a
chloride system dry, attempting to brute-force perchlorate from chloride, etc. and the mesh has stood up. He hooked me up with his supplier, and I
bought a sheet. Any failure mode we see will not be the underlying material... hopefully!
Thanks guys for the explanation of metathesis. I am getting dumb in my old age... It would make sense to use a small sample, and quantitatively
determine the chlorate content by weighing the crystallized product after conversion, then simply scaling up the procedure for the remainder of the
cell.
Question of the day: The "10% Rule." Traditionally, chlorate cells are halted when the chloride concentration reaches 10% by
weight, 100g/l chloride ion. There are two reasons, I believe, why this should be. The first is inefficiency, the cost of the electricity, which
will undoubtedly go up as chloride is depleted. The second is the wear and abuse the anode faces as chloride concentration drops.
If (and it's a big if) this MMO is as tough as I believe it to be, I am not overly concerned about wear or erosion, nor am I too concerned about the
electricity. I AM interested in maximizing yield per run, within reason. Given that a potassium-salted cell starts at, best case, only 14% chloride,
I'm having a tough time visualizing the cell struggling at 10%. That's only 28% of the chloride converted. I can visualize a cell beginning to gag a
bit when 60 to 75% of the chloride has been converted to chlorate.
Is my logic flawed?
I've been testing chloride concentration periodically throughout this run. Since the system is at a constant 50 amps, it might be interesting to plot
chloride conversion vs. time, and look at the shape of the curve. That will help visualize what is happening with regards to the rate and efficiency
of the conversion.
In retrospect, I wish I had executed more chloride concentration data points, but the Hach strips are not cheap and I'm being miserly with them.
[Edited on 12-11-2008 by Swede]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Xenoid: I don't have any data on the surface area ratio of this mesh, so I took the flat area of one side and multiplied by 1.5 and called it a
number. It's probably in the ballpark, and if I'm a bit low or a bit high, the plating should still have been near the sweet spot for beta-LD.
Swede: I don't see any reason why you can't dissolve the KCl at closer to cell operating temperature - at 40C, you can get about 194g/l chloride ion
into solution. That's not unreasonable, I think - just bring the electrolyte up to say 60C before you pour it in the cell, to warm up the container
nicely. At room temp you can get about 165g/l chloride ion in there, so it's not a huge difference, but it will certainly help. I think your 14%
chloride is perhaps derived from the 0C solubility? Or my math could be wrong in some significant way.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Tentacles, my solubility chart shows 350 grams per liter at 25 C. Chlorine is 47.5% by weight, so that would equal 166 g/l chloride, or 16.6% so I
think our math is correct. The odd thing is that even when I saturate boiling water, then let it cool down, and the excess crystallizes off, it seems
to measure 14 to 15% at 25C and no more.
Your point about putting the liquor in at 50 or 60, and having it carry the appropriate chloride with it, is a good one. But it can be tricky to do
with 5 to 10 gallons... got to heat up the liquor, get the extra KCl dissolved, then get it to operating temp before any KCl falls out as xtals.
Although I suppose if they did, they'd redissolve as the temp goes up, especially with some agitation available.
Or, execute a chloride ion replenishment rig! 
Speaking of solubilities, here's a handy chart with many compounds of interest...
http://www.saskschools.ca/curr_content/chem30_05/4_solutions/solution3_1.htm
I've got a few improvements in mind for the next run, eespecially in the realm of agitation. Simply sticking in a 1/4" PTFE tube and attaching an
aquarium air pump isn't hacking it. The tubes jam with crystals near the end of the run. I'm thinking a vertical, wide-bore PVC pipe, cross drilled,
and open at the bottom, suspended from the lid, located close to the electrodes where the heat would be greatest. No way that thing would clog.
[Edited on 12-11-2008 by Swede]
[Edited on 12-11-2008 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
The only thing I can add to the 100g per liter lower limit of Chloride in cells is:
There is a risk that Perchlorate may start to form (not a problem with MMO). This is considered a contaminant.
This amount of Chloride, together with the high conc. of Chlorate, may best suit the crystallizer end of of the process too.
You can probably go way below the '100g/l Chloride rule' with MMO if you so wish. Industry will be very concerned, as you
said, about voltage rises, etc which will not bother us.
It would be alot of work but if you were to set up a row of small opentop glass containers containing a range of
concentrations of Chloride. Into each you would place a drop of known concentration of Silver Nitrate and record
(with a photo or video) what the immediate ppt of white Silver Chloride looked like. This would give you (us all) a
permanent way to do a quick and dirty Chloride titration.
There is a useful patent below showing graphs and system for commercial K Chlorate production.
The graphs may be useful. They are not in % though which is what we would like.
Note the temperature of their cell is 75C.
@Tentacles.
The picture below shows your LD'ed MMO. The indents are caused by bubbles AFAIK. Are there as many
or any bubble indents on the top sides of the cross members of mesh (this picture is taken from the bottom
I presume).
A good shake of MMO now and again when plating would help to get rid of bubbles.
There is also a stealth bomber parked on the MMO close to the top lhs..............
For circulation, (of Chlorate cell) place a large dia. (suspend form lid or where ever) pipe over the electrodes going down to close to the bottom of
the cell. The top ot the pipe needs to be below the surface of the electrolyte. The H2 will circulate the electrolyte around using this pipe.
Dann2
[Edited on 12-11-2008 by dann2]

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Patent No. 4339312
Continous process for conversion of Potassium Chloride to Chlorate
Attachment: US4339312.pdf (294kB)
This file has been downloaded 869 times
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
dann: I think the bomber there is one of those government micro-spyplanes. Or a bit of fuzz from the paper towel I gently dried it with.
Alternatively, could just be a bit of PbO trapped in the PbO2.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
For fun, I've began plotting chloride concentration over time in a constant current setup. I'm using Hach chloride strips, diluted 50:1, using a good
pipette and distilled water. The Hach strips have performed admirably for quite a while now, and I trust their accuracy.
Unfortunately, early in the run, I skipped too many opportunities to plot, mainly because it is a bit of a PITA, and the strips are about a buck
apiece. I guess I'm cheap.
The starting concentration is low because I used the liquor left over from the aborted circulating run. I simply tossed it back into the monolithic
vat and applied the juice.
My plots so far: Time (hours) vs chloride (g/l), 50 amps, constant current
0 124.2
64.5 114.4
103 99
119 95
126 92.5
If you plot these few points, the curve is somewhat flat, then begins to nosedive. The "nosedive" portion, where I'm at right now, is roughly linear,
but it shows signs of flattening out. Something like this:

If I were to venture a guess, the upper portion of the curve is where I was refining pH control, and the correct ionic species were being created.
The chloride level begins to dive in the heart of the process, where everything is close to optimum. I suspect the curve will flatten as chloride
drops and inefficiency takes over a bit. The flatness of the curve, as I continue to take data, will determine when I will pull the plug on this run.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
Great piece of data from MMO run. The strips are definitely the biz! (price ignored).
What temperature has the cell stabilized at?
Perhaps MMO is not capable of taking the Chloride concentration low. The graph will soon be straight.
Dann2
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Something I meant to mention earlier, regarding the air lines clogging.. Perhaps running a length of it inside the chamber down to the bottom and back
up a bit might help? This would preheat the air a tad and offer cooler places in the cell where chlorate might precipitate. Could be that the PTFE
tubing won't conduct enough heat, and air doesn't have much heat capacity anyways.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@Dann2 - I wish I could continue with this run in particular, measuring chloride, and taking it very low, but I am having some real difficulty
maintaining circulation. The PTFE tubing is clogging every 2 hours or so, and the only way to clear it is to take compressed air from a large shop
compressor, and blow 20 to 30 PSI through the tube. At night, while I sleep, the tube is jammed for 6 hours at least, and I'm not comfortable
running 50 amps without some circulation. I've got some ideas for the next run.
What I'd like to do is a run or two with a very small, clear cell, maybe 2 liters, 10 - 15 amps, with this particular anode material, and gather some
serious data on pH, voltage, chloride concentration, etc. With a small cell, I can conclude the run quickly, get good data, which should scale well.
Timers: Looking at irrigation timers yesterday, many of them have a master relay port, designed to actuate a 24V pump relay. This means any time the
timer is ON, the relay port is energized. That would be the mechanism to turn on the dosing pump, rather than one of the branch circuits.
@Tentacles: That is a good thought on the PTFE tube. One other option might be to coil the tube around a form, apply enough heat to set the tubing
so that it retains the coiled shape, and that would create a lengthy path for the air to preheat a bit before it exhausts.
My sample port + valve is completely jammed as well:

I should have expected this, given how low on the cell it was placed. What I can do is create a curved standpipe that will pull liquor from the top
of the cell, route it to the existing port, and hopefully that will not jam as badly as this one. The location of the jam is the threaded fitting
installed in the wall of the cell; again, temperature gradients cause a premature crystallization in all the areas that are a bit cooler than the
heart of the cell.
I turned the current down to 3 amps this morning. The system is cooling as I sit here, and it should be ambient in a few hours. I'll harvest the
batch, and should get some interesting pics.
A plastic sieve used by the bio-diesel people, set on a 5 gallon pail, will make a handy crystal strainer:

Based upon my chloride ion measurements, I am estimating the yield at 2.5 kg. Could be better, but the starting liquor was weak.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by dann2
The picture below shows your LD'ed MMO. The indents are caused by bubbles AFAIK. Are there as many
or any bubble indents on the top sides of the cross members of mesh (this picture is taken from the bottom
I presume).
A good shake of MMO now and again when plating would help to get rid of bubbles. |
A pulsed power system might
help with bubble formation as well. Rather than using DC power, a pulse train is used. The simplest kind of pulse train is just some chopped duty
cycle, switching the DC on and off. The reason this is effective is that it changes the ionic balance near the electrode, as well as allowing
deadsorbtion of ionic species that are held only by electrostatic forces. Constant DC creates a depletion zone in the electrolyte near the electrode,
depleted of the species that are plating-on. Pulsing the voltage can allow quicker regeneration of this zone; regeneration is diffusion limited.
Plating rates can actually be higher at the same DC level. Fancier versions incorporate small reverse-polarity pulses to do more aggressive
modifications of the depletion area.
This can get rid of bubbles by rejecting the gas forming species at the ion level before they have time to nucleate and form a bubble.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
I wish I could continue with this run in particular, measuring chloride, and taking it very low, but I am having some real difficulty maintaining
circulation. |
Have you consider mechanical stirring? For a cell of your size (pretty small), glass is still
mechanically sound enough to be used as an axle. Melt on some paddles, pass the axle through a gasket at the top, and you're good to go. You don't
need borosilicate for this application (although it's fine), because there are no large temperature gradients around. Soda-lime glass can be worked
with propane/air. Glass isn't necessary, of course, anything that resists the oxidizing environment is sufficient.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by watson.fawkes
Have you consider mechanical stirring? For a cell of your size (pretty small), glass is still mechanically sound enough to be used as an axle. Melt on
some paddles, pass the axle through a gasket at the top, and you're good to go. You don't need borosilicate for this application (although it's fine),
because there are no large temperature gradients around. Soda-lime glass can be worked with propane/air. Glass isn't necessary, of course, anything
that resists the oxidizing environment is sufficient. |
watson.fawkes, I concur with your assessment. I popped the lid to T-Cell II to expedite cooling, and everything, materials-wise, looks good. There
is a nice layer of fat crystals in the bottom. But the PTFE tubes were hopelessly jammed. The CPVC acid diffusion down-tube was clear, including the
side-holes, so something similar would be the way to go with air, at least. Even if the side-holes jammed, I don't think it'd be physically possible
to clog the bottom of the pipe.
But back to mechanical stirring - the big benefit is lack of spatter. With my acrylic tower, where I could see what was going on, the air circulation
did work well, dispursing evolved electrode gases evenly, and keeping the temperature even as well, but as the bubbles broke the surface, they tossed
liquor all along the seam. Even with careful attention to sealing, this creates a serious salt creep that is very difficult to halt. An additional
negative - by introducing air, you are pressurizing the vessel a bit, exacerbating the problem.
A PTFE turning would make an ideal gasket for a mechanical stirrer, and a gearmotor, at perhaps 60 to 120 RPM, rotating paddles near the electrodes,
would do a fine job.
The grey PVC plastic was a bit bleached but otherwise sound. The sight glass, which I was so proud of, will have to be scrapped and replaced with
something having a much larger bore. I've got a ton of mental notes on improving this thing that I am going to incorporate into the next run. That's
one of the nice things about (C)PVC... it solvent welds, and can be modified easily.
With the top off, the cooling should accelerate, then it's time to harvest the crop. I've got some cool pics I hope you guys will enjoy. I've also
got some chems on the way, in an attempt to replicate Tentacles plate job. 
[Edited on 13-11-2008 by Swede]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
A PTFE turning would make an ideal gasket for a mechanical stirrer, and a gearmotor, at perhaps 60 to 120 RPM, rotating paddles near the electrodes,
would do a fine job. [...] That's one of the nice things about (C)PVC... it solvent welds, and can be modified easily. |
I might suggest, for the sake of easier rework, to turn your bushing to the O.D. of a standard pipe side and thread one end with
male pipe threads. Then glue in a female pipe thread fitting as the orifice. It would make reconfiguration that much faster, at the cost of slightly
greater up-front fabrication time.
In that vein, you could also provide a quick mount for a stirring motor. The brass inserts that screw into wood and provide a captured machine thread
can also be used in practice. Use a small-diameter, thick-walled pipe. Drill slightly undersize, heat up the insert on the end of a mandrel/bolt, and
screw it right in while hot. Solvent weld the pipe section where you want it. The pipe acts as an integral stand-off. Mount your motor to a plate.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
One alternative to this machining time (although I know you have PTFE barstock, Swede) would be to machine a bore and use PTFE o-rings as the seal.
I'm not sure what would be appropriate to lubricate with, however, but some sort of lubricant would definitely make the assembly last longer and seal
better. It needs to be stiff enough, or have a second bearing surface, to keep the stirrer from flopping all over.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
I still think my (fantasticly simple) circulation system is all that is required, perhaps not. It would not be capable of stirring up the layer of
crystals on the bottom which would sit there together with trapped fluid in their midst.
You won't beat it on price though!
A submersible pump would do the job too.
This one does 14 liter per minute and is cheap. It may not like the hot salty electrolyte.
http://cgi.ebay.co.uk/caravan-Submersible-water-pump-12-volt...
This might do:
http://cgi.ebay.com/12v-Submersible-Pump-Salt-Water-Diesel-K...
Or this outside if the salts don't plug the pipes.
http://cgi.ebay.com/IWAKI-CMD-228-PUMP-SALT-WATER-FISH-TANK-...
It's all money.
The motor + shaft + paddle +bearing/seal seems complicated and liable to wear and will generally give trouble in the medium to long term.
I guess you could have it on (another!) a timer so that it does not run 24/7.
Dann2
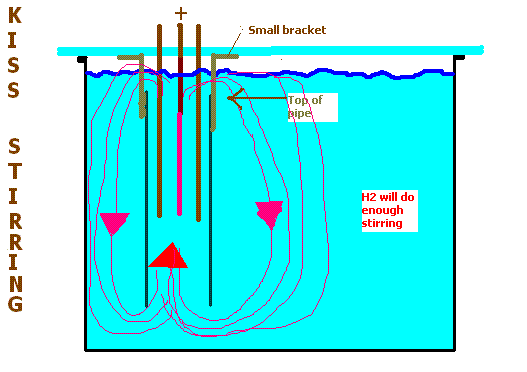
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Dann2, I think the circulation that your thermal (and H2) forces created by the setup in your drawing might be more than adequate - it'd be well worth
a try, perhaps on a small scale at first. The gasses and heat generated at 50 to 100 amps are not trivial, and if encased in a relatively narrow
pipe, I suspect the flow through the pipe might be measured at several liters per hour. There's a lot to be said for KISS. I am learning that lesson
well with my latest iteration.
About the only thing I'd do different from your drawing would be to seal the bottom of the pipe, and drill inlet holes in the sides of the pipe at the
base, around the perimeter... this might help keep the crystals at the bottom from being drawn up into the pipe.
I harvested a giant batch (for me) from my latest rig. More details than you probably want right here....
The crystal mass at the bottom was both thick and very firm. I had to beat it into manageable chunks with a plastic rod. Oddly, it had two distinct
layers:

The entire mass went into a bucket sieve for washing:

I've learned a lot from this run, and have some distinct improvements in mind for the next. I'm also more than ready to move onto perchlorate
production. Thank you all again for your expertise, and great suggestions.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Going back to the 'min 100 grams per liter rule' for Chloride, I think this is from the days of Graphite (and possible Pt too), where going below this
give non economic Graphite wear.
You could not run cells at higher temp. and low Cl- concentrations.
With DSA things seemed to have changed.
The attached patent is worth reading if time permits.
It states:
With the advent of DSA it has become possible to operate
cells up to boilig point and down to Chloride concs. of 30grams per liter and 700 grams per liter Chlorate.
You can then get a crop of Chlorate directly be evaporation and cooling.
With Graphite you would take a crop of Sodium Chloride out first then a small amount of Chlorate.
Two other patents showing phase diagrams for Chlorate removal are:
US3511619
US3690845
Dann2
Attachment: US3883406 Na Chlorate Phase.pdf (234kB)
This file has been downloaded 837 times
|
|
|
| Pages:
1
..
8
9
10
11
12
..
48 |
|