pH-meter Homemade "Details"
So i had successfully made some investigations and test in my lab in the subject, i'm very interested on looking forward to see what these can lead to
so here is everything of info i have to offer 
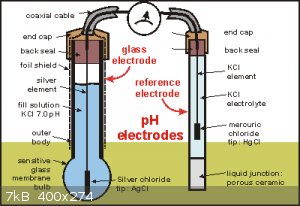
First how pH probes work, The famous glass electrode is a glass bulb that has a surface doped with silicon dioxide in both inside and outside, and
contains a internal electrode made from silver and coated with silver chloride. These are the Silver/Silver chloride type. Others use Mercury/Mercury
Chloride wich contains a paste made out of mercury, mercury chloride and potassium chloride anhydrous. The wire is a lead wire. now that is the first
mos important part, the second important part is the reference electrode. Its the same than the glass electrode, but these one has a permeable materia
in between the internal solution and the solution to titrate.
Ok so the chemical equilibrium associated are the next:
The silver wire and silver ions in solution:
Ag(Positive) + e --> Ag(Neutral)
AgCl ---> Ag(Positive) + Cl(Negative)
----------------------------------------------------------------
AgCl + e ---> Ag(Neutral) + Cl(Negative)
But i don't have any silver or silver chloride, so i had tried with others,
1) Copper/Cuprous chloride in sodium chloride: It gives very nice results but the downside is that the cuprous chloride is soluble in chloride
containing solution, hoever the life was something like a few minutes or so. Oxidation of the cuprous chloride also was a problem so i added a
reducing agent to try to maintain the cuprous in -1 oxidation state. But it ended up forming Chevreul's salt which was very interesting !!
2) Copper/Copper fluoride in sodium fluoride: This has also a very nice results, but i've had the problem that lectures are not so constant as with
the cuprous chloride, Although the cuprous chloride slowly dissolved out, it was more constant than the fluoride. And the fluoride also dissolved
away.
3) (The pictures were taken with these method)
Copper/Cuprous bromide in ammonium bromide. I know that the PH of the reference solution has to be in a pH closer to 7 as possible, but you get the
idea. These had lecture very constant, and also last longer than the other ones. One problem was the cuprous bromide in the copper wire used to fall
apart.
Ok, the most important part is the treatment of the glass electrode, i had tried with a number of techniques,
1) Putting the glass bulb in molten NaOH and then treated with hydrochloric acid to form the hydrate and flame it so the oxide layer was formed. Its
tricky because if you put it to long in the molten NaOH you will be left with no glass, ant to short the layer wil not be as thin as to give rapid
readings.
2) Same as in 1) both in both inside and outside of the glass bulb, it was very annoying but is the best you can do,
I dont have pictures of these because the ceramic container unfortunately does not exist any more. (-.-) BUT IM PLANNING TO POST A NEW TOPIC IN GLASS
ELECTRODES FABRICATION SO ALL THE INFO WILL BE IN THAT OTHER TOPIC.
Ok i hope you find this entertaining
|