Octavian
Harmless

Posts: 19
Registered: 25-5-2015
Member Is Offline
Mood: No Mood
|
|
Purification of benzaldehyde?
I've been given a small amount of benzaldehyde and have been having trouble distilling it, possibly due to my crappy aspirator. Seems that when I try
to vac distil it it refuses to come over and then once the oil bath hits around 150C it starts turning darker until i'm left with a black tar in my
flask that still refuses to come over even if the bath is taken up to 180C.
Since for the moment I don't have the spare money to drop on a cheap rotary vane pump setup, I was wondering if I could get away with just steam
distilling it? What I am thinking is strip off the low boiling fraction under vacuum then switch to a steam setup similar to the image attached. Once
all of the oil has crossed over, drain off the oil using a sep funnel then salt out the water to free what is dissolved in it, followed by drying the
benzaldehyde over MgSO4 - Would this work?
I'll be using a 500ml E-Flask for the steam generator, which i'm thinking is rather small for say 150ml - 200ml of benzaldehyde but it's the biggest I
currently have, I also plan on using a propane burner to super heat the steam just before it enters the RB flask which will be sitting in either a
water or oil bath at about 80C, can anyone see any problems with this?
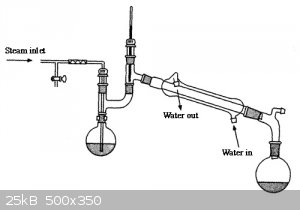
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
| Quote: | Benzaldehyde [100-52-7] m.p. -57°C, M 106.1, b.p. 62°C (58°C)/10mm, 179.0°C/760mm, d 1.044.
To diminish its rate of oxidation, benzaldehyde usually contains additives such as hydroquinone or catechol. It can be purified via its bisulfite
addition compound but usually distn (under nitrogen at reduced pressure) is sufficient. Prior to distn it is washed with NaOH or 10% Na2CO3 (until no
more CO2 is evolved), then with satd Na2SO3 and H2O, followed by drying with CaSO4, MgSO4 or CaCl2. |
Boiling point of benzaldehyde is close to 100°C at 100 mmHg, and at 200 mmHg it boils at 125°C. If you can't reach 200 mmHg with your vacuum device,
then it's useless. My aspirator easily reaches 200, and usually it's 120-150 mmHg, which is enought for most purposes.
UPD: fixed melting point info
[Edited on 26-5-2015 by byko3y]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This may prove useful:
http://www.sciencemadness.org/talk/viewthread.php?tid=26075
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Octavian
Harmless

Posts: 19
Registered: 25-5-2015
Member Is Offline
Mood: No Mood
|
|
byko3y: Not sure where you get your info but at 100torr it should be boiling at around 101C, the melting point for benzaldehyde is -57C, a difference
of 158C but yes, at 200torr (which my aspirator is capable of) it should be coming over at around 125C, the problem is that it won't  . I will get an occasional bump but that's it. If anyone has any insight as to why this
is happening it would be greatly appreciated. I was using mag stirring w/ the flask 3/4 submerged in an oil bath. . I will get an occasional bump but that's it. If anyone has any insight as to why this
is happening it would be greatly appreciated. I was using mag stirring w/ the flask 3/4 submerged in an oil bath.
Thanks for the link Magpie! I have attempted internal steam distillation before but it was a very slow process, I was hoping that maybe generating the
steam externally would speed the process up a bit?
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Do you have strongs bumps? If so, then overheating is your problem and you need some way of making it boil.
|
|
|
Octavian
Harmless

Posts: 19
Registered: 25-5-2015
Member Is Offline
Mood: No Mood
|
|
Yep, it will sit there not doing anything then one or two violent bumps then nothing for another minute or two. Any ideas how I could get it to boil?
I figured that mag stirring would have been enough but apparently not. This is the first high boiling liquid i've had to distil so i'm still very much
a n00b. I've distilled HNO3, MeOH, EtOH, Shellite, MEK, Acetone and water before without an issue but that is the extent of my experience.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
An ebulliator should stop that violent bumping. Use of one is usually needed for vacuum distillations.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Octavian
Harmless

Posts: 19
Registered: 25-5-2015
Member Is Offline
Mood: No Mood
|
|
I had to google "ebulliator", first time I've heard that term. I thought a ebulliator or capillary was only needed when magnetic stirring wasn't
possible? I did try drawing a capillary using 6mm glass tubing before i made a stirring oil bath however the benzaldehyde still didn't come over and
of course degraded quite quickly due to having air passed through it while being heated.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2660
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I don't know that benzaldehyde is an ideal steam distillation candidate. I would try to get the best vacuum that you can, and distill it quickly,
the main impurity will be benzoic acid (from air oxidation). Alternatively, you could just dissolve it in ether or a similar solvent and wash with
1N NaOH to remove the benzoic acid. And let the apparatus cool down a while before releasing the vacuum, hot benzaldehyde will oxidize even faster
when exposed to air.
The key to avoiding bumping is to heat all around the sides of the rbf, not just the bottom in just one spot, which is what I saw many people do in
org. chem classes.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Distilling benzaldehyde is a pain. I'd wash it with 10% sodium carbonate (this gets rid of the benzoic acid, the crystalline stuff in old bottles) and
dry it (MgSO4 and a trace of TBHQ or BHT to prevent further autooxidation).
If you need purer material, still, you can extract it with aqueous sodium metabisulfite to make the organosulfite. The aqueous solution is then
treated with acid or base (acid is typically preferred, I've had good results with alkali, but you must watch the pH) to release the aldehyde, which
should float. Then dry/preserve.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Of course, you can steam distill it. Benzaldehyde is sometimes produced, by steam distilling it from natural products. One method, involves basic
hydrolysis (reverse aldol) and steam distillation of Benzaldehyde from Cinnamaldehyde. Examples can be found via the search engine.
Get a vacuum pump. Benzaldehyde can still be obtained, but no reagent is really cheap nowadays. And , it is a shame to waste Benzaldehyde,
especially since it is a listed material.
I bought a pretty slick pump at Harbor Freight a little while back. With a discount coupon, it was under $120.00.. Pulls a hell-acious vacuum.
Just be sure you change the oil often and use traps to protect it from corrosives.
|
|
|