Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
ONTA
I`ve read something about ONTA, oxynitrotriazole and have no infos to the exact formula of the explosive. Someone can tell more to that or any reply
will help. I hope i will found a way to a useful synthesis wich will work.
Thanks
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
NTO is easier. Nitrourea reduction with acid/Zn, followed by precipitation of the semicarbazide produced with acetone as acetone semicarbazone,
followed by hydrolysis and pptation of the semicarbazide with HCl, followed by reaction with formic acid (limescale remover is a common OTC source) to
form triazolone. Heating this with 70% nitric yields NTO (3-nitro-1,2,4-triazol-5-one) in good yield.
It's insensitive, and about as powerful as RDX. I have lots of information on my home computer, but I'm currently away. I have prepared NTO from
commercial semicarbazide hydrochloride, I didn't get a good yield when attempting to make semicarbazide myself, but then, I think I only tried once.
Semicarbazide can also be made from urea and hydrazine, and even directly from urea and hypochlorite, with the formation of hydrazine in situ.
Oh, hang on, you're probably talking about NTO, but using a less common name! So what was I thinking of..? There is another similarly structured
compound... Ah yes, I was thinking of ANTA, 3-amino-5-nitro-1,2,4-triazole. Which seems almost identical in its properties...
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
I'd love to hear more details on the synthesis, etc. of NTO whenever you get a chance.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
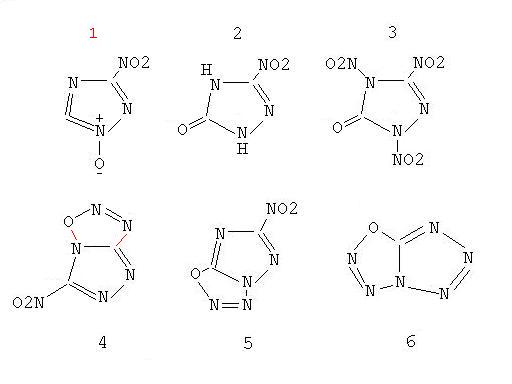
Thaks that you had gave some to nitrotriazole. I have made a small pic to the topic.
I guess NTO is nitrated in 99% HNO3 and N2O5, this will give 1,2,4-trinitrotriazole-5-one
in figure 3. I have found more to that. Oxinitrotriazole or 3-nitrotriazole-n-oxide will show
the picture in figure 1. I don`t have found some to that but it will have a "chance", possibly
anyone found more to this. I`ve chosen some more and that will show the pictures in figure 3 to 6.
It will show some to obtain useful nitrodiazooxides.
1-amino-3-nitro-5-hydroxy-1,2,4-triazole in figure 5 will give 3-nitro-1,2,4-triazole-1-diazo-5-oxide
and possibly 5-hydroxy-1-amino-tetrazole will give
5-diazotetrazole-1-oxide. I don`t know wich stuff are needed to begin the syntheses to that but i think
it have any interest. This will take some time and the theoretical method is not correct always
like the known method when the product is soluble in H2O, can dissolved in dilute H2SO4 or HCl and than
combinued with a solution of NaNO2/H2O. Excuse me, this is not made between some days.
I`ve reworked the JPG and hoped the mistake in figure 1 was seen by anyone.
I`ve found the mentioned oxinitrotriatole in a document wich was listened explosives
of WKII and their storage stability. I guess it will be the explosive in figure 1 or the
document will describe wrong details unless it is still a triazole or it have not a practical
importance. I don`t know 3-nitrotriazole will be a explosive or it is rather hard to detonate.
A perchlorate of 3-aminotriazole or his salt is a good idea.
Any methods will describe this. I hope it will work.
Prepare a perchlorate salt by a chloride salt and perchloric acid, the dryed salt is than dissolved in
water and this is than stirred to the a solution of 3-aminotriazole and perchloric acid.
Some more nitrotriazoles are 3,5-dinitrotriazole, 1,3,5-trinitrotriazole and 3-amino-1,5-dinitrotriazole wich will be
obtained by concentrated HNO3 and HNO3/P2O5.
I`ve searched sometimes and i guess that lower listened tetrazoles will have any interest to begin a useful synthesis
to some explosives.
5-Aminotetrazole monohydrate, CAS Number: 15454-54-3
1-AMINO-4,5-DIHYDRO-1H-TETRAZOL-5-ONE, CAS Number: 84143-61-3
[Edited on 3-8-2006 by Mason_Grand_ANNdrews]
[Edited on 3-8-2006 by Mason_Grand_ANNdrews]
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
There's still a mistake in figure 1 - you've drawn an sp2 hybridised carbon without a 180* bond angle.
Figure 2 is NTO. Nitrating it to 3,4-dinitro-1,2,4-triazol-5-one might not be too difficult, but getting a nitro group on the N at position 1 as well,
to give what is shown in figure 3, won't be as easy because the lone pair of that nitrogen is conjugated with both the carbonyl and nitro group at
carbon 3, and the inductive effect from the nitro group on the N at position 4 will also make the N at position 1 much less nucleophilic.
3,4-dinitro-1,2,4-triazol-5-one might be interesting too, because it's probably quite acidic and so might form nice primary salts (silver, lead,
mercury).
Figure 6 won't be practical - things like that explode spontaneously, even in dilute solutions in some cases!
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
What is told, i don`t know it would be a practical way for a synthesis of a n-oxide of a triazole.
A practical method for a n-oxide is hard to get. I`ve searched and found some variably n-bonds.
isoquinoline-n-oxide
methypyridine-1-oxide
I don`t know a similar n-oxide than isoquinoline-n-oxide is interested obtain such a nitrotriazole. A explosive is stable, only a practical test will
show that.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
After some time i have firgured out some new suggetsions to the theme triazoles and tetrazoles. I guess some of the ideas are very interested. If
there some errors in the draws or anything that needs correcting then please comment.
I hope anyone have some practical issues to the compounds.
[Edited on 16-9-2006 by Mason_Grand_ANNdrews]
Attachment: triazoles-tetrazoles.zip (390kB)
This file has been downloaded 630 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
NTO synthesis and research paper
Here's a paper which describes more about NTO.
http://dspace.dsto.defence.gov.au/dspace/bitstream/1947/3884...
alternate link for same paper
http://docs.google.com/viewer?a=v&q=cache:6BOE8OIloK8J:d...
[Edited on 17-9-2010 by Rosco Bodine]
|
|
|