Thaekross
Harmless

Posts: 17
Registered: 28-7-2015
Location: Inner-most circle of Hell
Member Is Offline
Mood: Neutral and utterly so
|
|
Help with indole to substituted tryptamines.
"The indolylmagnesium iodide reagent was prepared in the usual manner from an indole and ethylmagnesium iodide in dry anisole at 10°C. The mixture
was cooled to -5°C and a solution of 2-molar equivalents of β-dimethylaminoethyl chloride in dry benzene was added. After 8 hours at -5°C the
mixture was allowed to warm slowly to room temperature, left overnight, and then added to aqueous ammonium chloride. The product was obtained via
extraction into dilute hydrochloric acid and subsequent neutralisation."
This reaction I found very interesting as it doesn't seem to produce any yield in THF or ether and not with other metals. I'm wondering if carbon-3
alkylation might be possible with other alkyl-magnesium halides (e.g. ethylmagnesium chloride/bromide) or if it would only work (with decent yield)
with iodine, due to how proficient iodine is as a leaving group. I could be wrong, however.
Interesting chemistry, nonetheless.
Reference: https://www.erowid.org/archive/rhodium/chemistry/dmt.indole....
More so; A translated reference from Bull soc chim Fr 1961, 1190:
"Experimental
To 15.6g (0.13 mole) of methyl-magnesium bromide in ether is added 15.3g of indole (0.13 mole) in ether and then 5.6g of ethylene-imine in anhydrous
xylene. The ether is eliminated completely by distillation while some xylene is added. The mixture is refluxed until all become solid. This is
decomposed by addition of cold water, then some concentrated HCl is added till pH 1 and the tryptamine chlorydrate is separated. The freebase is
liberated from the addition of an aqueous solution of NaOH. Yield = 9.6g (46%) mp= 116°C"
Though I don't think I can use this to produce substituted tryptamines, due to the starting use of a secondary amine (ethylene-imine [aziridine]) to
produce the primary amine (tryptamine).
Insight of reaction mechanisms would be awesome! I find this field particularly interesting (for some unknown reason).
Any corrections and suggestions are absolutely welcome; I know I'm not the most well versed of us. Really need to read up on electronegativity and
nucleophilicity 
Okay, so think of it like this: It's the part of existence that makes different things happen. I guess you could call it the first thing other than
nothing.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Unfortunately I can't give either corrections or suggestions but have a question. :-)
How come aziridine doesn't destroy one equivalent of the Grignard reagent immediately upon addition? As I looked it up it has an N-H bond and I always
thought that such bonds mean "active hydrogen", at least active against such basic compounds as a Grignard.
Shouldn't it produce methane from the methyl-magnesium-bromide?
|
|
|
Thaekross
Harmless

Posts: 17
Registered: 28-7-2015
Location: Inner-most circle of Hell
Member Is Offline
Mood: Neutral and utterly so
|
|
It does produce methane, as far as I know, but the reaction "transfers" the magnesium halide to the nitrogen, producing indolylmagnesium halide which
is usually protonated to the product in a usual Grignard reaction. In this case, the protonation is skipped and the intermediate is used in a second
reaction  (This is where the aziridine reacts as it is added after) (This is where the aziridine reacts as it is added after)
[Edited on 6-9-2015 by Thaekross]
Okay, so think of it like this: It's the part of existence that makes different things happen. I guess you could call it the first thing other than
nothing.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I don't really understand the reason why cold anisole is the only possible solvent for the reaction. Those guys promised to describe the details of
the reaction conditions but I have not found such an article yet (it might be non-existent  ). ).
But they did prepare some more compounds using this method.
Aminoalkylation of metal derivatives of indole. Part II. Coupling of indolylmagnesium iodides with halogenoalkylamines
The reaction is pretty much a Stork enamine alkylation which was descovered relatively recently by guess who A New Method for the Alkylation of Ketones and Aldehydes: the C-Alkylation of the Magnesium Salts of N-Substituted Imines
There are more examples in the literature, and in these cases neither anisole, nor low temperature were used.
Reaction of indolylmagnesium bromide and chloromethylpyridines. Synthesis of skatylpyridines and piperidines
Synthesis of Substituted-(l)-Tryptophanols from an Enantiomerically Pure Aziridine-2-methanol
And the modern procedure from 1961 mentioned by you also uses xylene and high temperature for the similar preparation - pyrrolidine cyclization is a
main side product there AFAIK.
| Quote: | | How come aziridine doesn't destroy one equivalent of the Grignard reagent immediately upon addition? |
This
also might be a reason for lower yields. Although I don't understand why tertiary amine also causes a lot of troubles while having no hydrogens
attached to its nitrogen.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
| Quote: | Sebastian also observed that although alkylation of the indole Grignard reagent with methyl iodide in tetrahydrofuran at 23°C gave essentially
3-methylindole, variable amounts of 1- and 3-methyl-indole were obtained on alkylation of the alkali metal salts of indole under similar conditions.
Sebastian's results were qualitatively similar to those obtained earlier by Lerner more recently by Cardillo et al. who studied the reaction of a
number of organometallic derivatives of indole, including the indole Grignard reagents, with allyl bromide. N-Substitution was favored by
increasing electropositivity of the cation, i.e., relatively more N-substitution was observed in the case of the potassium derivative than in the
case of the lithium. Furthermore, factors that tended to facilitate dissociation of the indole salts, such as increasing the polarity of the medium,
increased the tendency for substitution to occur at the 1-position....
Powers et al. concluded, as a result of their investigations of the protonation of the indole Grignard reagent, that in ether solution, at least, the
N-MgX bond of the indole Grignard reagent has a considerable covalent character. In tetrahydrofuran, however, the stronger basicity of this ether,
which would coordinate more strongly with the magnesium, would increase the ionic character of the N-MgX bond.
By virtue of their extensive studies on the interaction of β-dimethyl-aminoalkyl halides with some organometallic derivatives of indole, induding the
indole Grignard reagents, Ganellin and Ridley came to conclusions not too dissimilar to those of Sebastian and Powers et al. These workers were able
to exclude the possibility of a C-MgX species and concluded that the indole Grignard reagents are essentially N-MgX species in which the N-MgX bond in
the indole magnesium halides is formally covalent but is polarized to induce partial anionic character in the indole nucleus, with the consequent
increase in electron density at the 3-position, which would facilitate electrophilic substitution at that position. |
"The Indole Grignard Reagents"
So here's the anwer - reaction works fine until you use a polar solvent, and diethyl ether is also a relatively polar solvent.
[Edited on 6-9-2015 by byko3y]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Thanks Thaekross, I went on a mandatory field trip in the morning after posting my 2 cents, but had time to ponder on this reaction. :-)
I realised that indole also has an active H on its nitrogen and although I did not look it up but I think that H is much more acidic than the H in
aziridine. So you are right, at first indole "consumes" the Grignard reagent and yields a Mg salt, then this salt is used in the ring-opening addition
of aziridine to yield tryptamine.
(Btw. 5.6g ethylene-imine is just about 0.13 mole.)
|
|
|
zed
International Hazard
    
Posts: 2278
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
My buddy Dr. Death used to run this reaction. The original authors tried lots of solvents. Only Anisole produced good yields.
Upon the addition of EthylMagnesiumIodide to Indole. An Indolyl-MagnesiumIodide complex is formed. Ethane gas exits. So be ready for that
eventuality.
It is also important to note, that constant stirring is required, to produce good yields. And, ordinary stirring, will not be sufficient. Your
reaction complex will attempt to
become a solid block of crud. Your battle will be to keep it slurry. A hefty Low-speed DC motor, or an Air-Motor, may be required to accomplish the
task.
I recall seeing an account of a variant of this reaction, wherein EthylMagnesium Iodide, and DimethylaminoEthylChloride, are dripped simultaneous
into an Indole solution, to achieve the same synthetic result.
[Edited on 6-9-2015 by zed]
|
|
|
Thaekross
Harmless

Posts: 17
Registered: 28-7-2015
Location: Inner-most circle of Hell
Member Is Offline
Mood: Neutral and utterly so
|
|
I'm glad this is promoting some thinking!
Thank you, byko3y, for your Ref. on the use of ether  Unfortunate, as ether is
kinda my go-to guy, but I see how it is not suited now that you mentioned it. That quote did wonders at explaining the mechanism too. Unfortunate, as ether is
kinda my go-to guy, but I see how it is not suited now that you mentioned it. That quote did wonders at explaining the mechanism too.
zed, would you be able to recall how long Dr. Death ran this reaction?
From what I can tell, this should be possible with the use of other halide as long as the right solvent is used and time is allowed for the evolution
of which ever alkane gas to cease before the addition of 2-Chloro-N,N-dialkylethylamine.
Okay, so think of it like this: It's the part of existence that makes different things happen. I guess you could call it the first thing other than
nothing.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
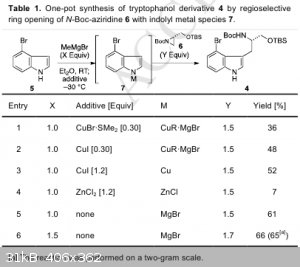
| Quote: | | A flame-dried 100-mL round-bottomed flask equipped with a Teflon-coated magnetic stirring bar, an inlet adapter with three-way stopcock was charged
with 4-bromoindole (5) (1.93 g, 9.83 mmol) and dry ether (4.3 mL). To the solution was added MeMgBr (3.0 M in ether, 4.26 mL, 12.8 mmol) at room
temperature. After the mixture was stirred at room temperature for 1.5 h, the mixture was added 6 (4.80 g, 16.7 mmol) in dry ether (10 mL). The
reaction mixture was stirred at room temperature for 6 h, after which time TLC (hexanes-ethyl acetate = 3:1) indicated complete consumption of
aziridine 6. The reaction was quenched with saturated aqueous NH4Cl. The aqueous layer was extracted with ether three times. The combined organic
extracts were washed with brine, dried over anhydrous sodium sulfate, and filtered. The organic solvents were removed under reduced pressure. The
residue was purified by silica gel column chromatography (hexanes-ethyl acetate = 4:1) to afford tryptophanol 4 (3.09 g, 6.38 mmol, 65%) as a
colorless amorphous. |
A Concise Total Synthesis of (–)-Indolactam V
doi:10.1016/j.tet.2015.04.015
|
|
|
Thaekross
Harmless

Posts: 17
Registered: 28-7-2015
Location: Inner-most circle of Hell
Member Is Offline
Mood: Neutral and utterly so
|
|
Thank you, byko3y. Useful Ref. 
If I get around to it I will post yields for EtMgCl to compare to Lit.
Okay, so think of it like this: It's the part of existence that makes different things happen. I guess you could call it the first thing other than
nothing.
|
|
|
zed
International Hazard
    
Posts: 2278
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ethyl Iodide is popular because of its BP, and reactivity. Expensive though. Sadly, you may find other Alkyl Halides more difficult to work with.
Either because they are not as reactive in forming Grignard reagents, or because they are gases at STP.
Dr. Death, stirred the slurry overnight.
Within the original article, there are references to other papers. Obtain copies of those papers, and read them. All will be explained.
The reaction sequence does work, and it does produce a yield of about 25%.
[Edited on 9-9-2015 by zed]
|
|
|