madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Willgerodt Reaction
<b>This post is an excerpt from an advanced organic chemistry textbook.</b>
ArCOCH<sub>3</sub> ----((NH<sub>4</sub> <sub>2</sub>S<sub>x</sub> <sub>2</sub>S<sub>x</sub> ---->
ArCH<sub>2</sub>CONH<sub>2</sub> + ArCH<sub>2</sub>COO<sup>-</sup>
NH<sub>4</sub><sup>+</sup> ---->
ArCH<sub>2</sub>CONH<sub>2</sub> + ArCH<sub>2</sub>COO<sup>-</sup>
NH<sub>4</sub><sup>+</sup>
In the <i>Willgerodt reaction</i> a straight- or branched-chain aryl alkyl ketone is converted to the amide and/or the ammonium salt of
the acid. The carbonyl group of the product is always at the end of the chain. Thus ArCOCH<sub>2</sub>CH<sub>3</sub> gives the
amide and the salt of ArCH<sub>2</sub>CH<sub>2</sub>COOH, and
ArCOCH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> gives derivatives of
ArCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>COOH. However, yields sharply decrease with increasing length of
chain. The reaction has also been carried out on vinyl and ethynyl aromatic compounds and on aliphatic ketones, but yields are usually lower in these
cases. The use of sulfur and a dry primary or secondary amine (or ammonia) as the reagent is called the <i>Kindler modification</i> of the
Willgerodt reaction. The product in this case is Ar(CH<sub>2</sub> <sub>n</sub>CSNR<sub>2</sub>, which may be hydrolyzed to the acid. <sub>n</sub>CSNR<sub>2</sub>, which may be hydrolyzed to the acid.
Though the mechanism of the Willgerodt reaction is not completely known, some conceivable mechanisms may be excluded by the facts which are known.
Thus, one might suppose that the alkyl group becomes completely detached from the ring and then attacks it with its other end. However, this
possibility is ruled out by experiments such as the following: when isobutyl phenyl ketone (<b>26</b> is subjected to the Willgerodt reaction, the product is <b>27</b>, and not <b>28</b>, which
would arise if the end carbon of the ketone became bonded to the ring in the product. is subjected to the Willgerodt reaction, the product is <b>27</b>, and not <b>28</b>, which
would arise if the end carbon of the ketone became bonded to the ring in the product.
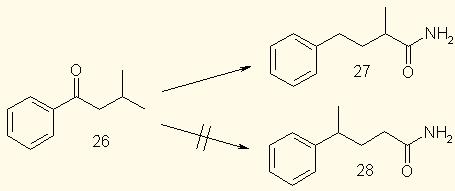
This also excludes a cyclic-intermediate mechanism, similar to that of the Claisen rearrangement. Another important fact is that the reaction is
successful for singly branched side chains, such as <b>26</b>, but not for doubly branched side chains, as in
PhCOCMe<sub>3</sub>. Still another piece of evidence is that compounds oxygenated along the chain give the same products; thus
PhCOCH<sub>2</sub>CH<sub>3</sub>, PhCH<sub>2</sub>COMe, and
PhCH<sub>2</sub>CH<sub>2</sub>CHO all give PhCH<sub>2</sub>CH<sub>2</sub>CONH<sub>2</sub>.
All of these facts point to a mechanism consisting of consecutive oxidations and reductions along the chain. Just what form these take is not certain,
though a number of theories have been proposed. Initial reduction to the hydrocarbon may be ruled out, since alkylbenzenes do not give the reaction.
When acetophenone was treated with butylamine and sulfur, in the Kindler procedure, the imine PhCMe=NBu could be isolated and then converted to the
normal product, indicating that the first step of the reaction may well be formation of the imine. The key steps of the mechanism may be of the type

<b>29</b> may be formed directly from the ketone, or from previously formed imine.
[Edited on 19-4-2003 by madscientist]
I weep at the sight of flaming acetic anhydride.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Quite a useful synthetic reaction! Some of us probably immediately thought "phenylacetic acid!" upon sighting of that first equation. The
Willgerodt reaction apparently provides a relatively easy route to phenylacetic acid - preparing acetophenone by destructive distillation of calcium
acetate/benzoate, then treating with ammonium polysulfide solution (prepared by adding sulfur to ammonia and warming), then heating with dilute
sulfuric acid (to completely hydrolysis and liberate the free acid). 
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Not to take you down, but I prefer the sweet smell of methylamine a 10.000 times over the the unbelievable stink of phenylacetic acid.
It is dependant on personal taste. Some say it stinks not a bit or a little bit and others - they run.
I am one of the second kind.
Ah! Methylamine! Sweet, sweet..... 
In my eyes phenylacetic acid is far overestimated. Comes because it was still easy available in the 80´s after P2P got regulated. But know what?
Until 1984 you could go to Switzerland enter a pharmacy and order and get some hundred grams of 1-phenyl-2-aminopropane without any problems for it
was plain legal. This fact was a driving force in cycle races like the Tour de France.... 
[Edited on 22-4-2003 by Organikum]
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
The text from where the information was taken was March's Advanced Organic Chemistry 5th edition ....page 1567 section 19-63, I bring this up
solely because as I ran out of reading on chapter 19, of the same book, this was the last entry and it contains many valuable references for the
reader to follow up.....I thought of posting the reaction, but first doing a search I found this thread........thanks to Madchemist as he prudently
saw the value of this reaction and posted it.........................solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It should be noted that styrene works, although not as well as acetophenone. Ordinary alphatic ketones also undergo the reaction, but usually with
lower yields.
Also note that the orginal Willgerodt - aq ammonia and sulfer - needs to be run under pressure, else the ammonia and water depart. A refinement is to
use a high boiling amine, morpholine was the one of choice long ago, and no water. I've heard of the use of hexamethylenediamine, from hydrolysis of
nylon, with a little butyl cellosolve; I wonder if the terminal -OH wouldn't get oxidized by the process.
|
|
|