NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Pyrazoline synthesis
Hello! I'm trying to make a 1,3,5-triaryl pirazoline from the condensation of a chalcone (1,3-diaryl-2-propenone) and benzoic hydrazide.
I tried 4 methods: in refluxing GAA (as catalyst and solvent), refluxing ethanol with a few drops of AcOH, in the microwave with AcOH and in the MW
with GAA and ammonium acetate. None of them is working, no product is formed. I am very disappointed 
What would you suggest me to do???
btw, the machanism is not quite elucidated. It is thought that the NH2 of the hydrazide attacks the carbonyl first, as no open product was isolated in
the literature, as far as i know.
Bromine, definitely bromine.
|
|
|
Boffis
International Hazard
    
Posts: 1843
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@NexusDNA, Can you provide us with a reference please. Some years ago I did some work on substituted pyrazolone from beta-ketoesters and substituted
phenylhydazines following a paper from a couple of Indian authors and while the simple ones worked I found the heavily substitute one difficult or
impossible to cyclotise.
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
RAGHAV, N. GARG, S. N-formylpyrazolines and N-benzoylpyrazolines as novel inhibitors of mammalian cathepsin B and cathepsin H. Bioorganic
Chemistry, 2014.
SRINIVAS, M. et al. Microwave adsisted synthesis and antimicrobial activity of some novel quinoxaline compounds. India: Mother Teresa
College of Pharmacy, 2013.
first one is themal, second one MW. In my case I'm using 1-(3,5-dimethoxy-phenyl)-3-(2-naphtyl)-propenone.
Bromine, definitely bromine.
|
|
|
Boffis
International Hazard
    
Posts: 1843
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Nexus, Let me look into these refs.
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
btw an update: I got it going, and it is beaaaautiful!!  

Bromine, definitely bromine.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Gorgeous color. Reminds me of 2,4-dinitrophenylhydrazine which is a bit darker and more wine-ish
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Did you do this with 1,3-diaryl-2-propenone and benzoic hydrazide? I'm not familiar with this cyclization. The ones I do require strong base. Did you
insert a catalyst? Does it cyclize due to stearic and energetic factors? Like everybody else I love the color! All my products are white and bitter.
What does it taste like? Pyrazoline looks like a very interesting intermediate for medicinal chemistry. I would like to make an acyl halide from the
stuff. No telling what wonderful activity might be evolved.
@mods: if words like halide aren't in our dictionary could we at least have an "ignore" option in the spell check? Maybe some kind person could edit
the dictionary so that chemical terms aren't underlined with color of UC235's product. Congratulations UC! Good job.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
N' methoxy pyrazinyl-3,4-methylenedioxyphenylpropanamine anyone?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Sorry for the late reply!
It doesnt require strong catalysts at all, in the literature they use GAA as a mild acid catalyst. I could not obtain the desired product with benzoic
hydrazide, so i broke the procedure in two steps: reacting the chalcone with hydrazine hydrate and then reacting the formed pyrazoline with benzoyl
chloride.
side note: I've seen some articles in which they test N-tosyl derivatives (instead of benzoyl chloride, TsCl) as potential MAO inhibitors with
excellent results. I won't try bioassaying my product though!! 
Here is the mechanism though to be operative:
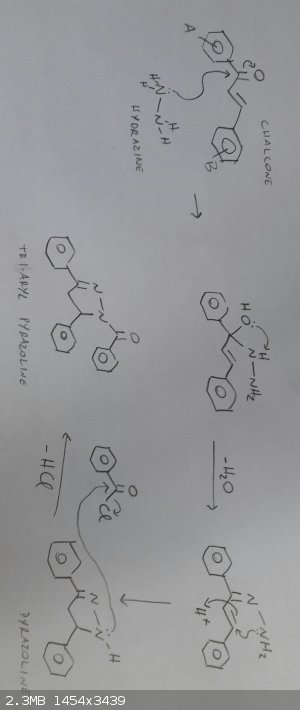
[Edited on 18-12-2015 by NexusDNA]
Bromine, definitely bromine.
|
|
|