ScienceBum
Harmless

Posts: 22
Registered: 15-9-2014
Location: Florida/California/Arizona
Member Is Offline
Mood: decoherenced
|
|
Synthesis of Natural Smells
Hey all, I was having a discussion with a chemist friend of mine about how some natural smells can be produced in the lab. I'm told that it's an
incredibly difficult not-worth-it venture requiring knowledge of complex organic chemistry, but I was wondering if anyone here was familiar with any
smell syntheses you could do in a home lab?
In particular I am interested in isolating the smell of various fruits such as strawberries(my personal favorite), apples and oranges. I do know that
for strawberries there is a compound that emulates some of the many compounds responsible for their smell, called furaneol but I have been
unable to find very much information on it's synthesis.
So if you guys know of any information regarding this subject, like if it is completely beyond the capabilities of a home lab or if you need special
equipment or anything, please let me know! and thanks!
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
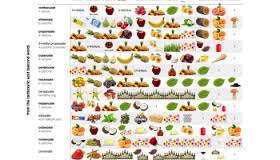
"Oil of Wintergreen" (methyl salicylate) is a very popular ester for high school chem labs. It can easily be made by esterify-ing salicylic acid with
methanol in an acidic solution. There are thousands of different smells that can be synthesized in the lab. Start with this table, pick a smell,
then research the synthesis.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Here's a link to that photo (it seems a bit small) and another similar table
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
ScienceBum
Harmless

Posts: 22
Registered: 15-9-2014
Location: Florida/California/Arizona
Member Is Offline
Mood: decoherenced
|
|
Ah thanks guys, I appreciate that  Just out of curiosity though, wikipedia says
that there's over 80-something compounds responsible for various fruit smells and flavors and that table only lists one ester per fruit from what I
can see. Is this basically the equivalent of comparing food flavoring additive to the actual food, or are these compounds the "main" ones responsible
for the smell? Just out of curiosity though, wikipedia says
that there's over 80-something compounds responsible for various fruit smells and flavors and that table only lists one ester per fruit from what I
can see. Is this basically the equivalent of comparing food flavoring additive to the actual food, or are these compounds the "main" ones responsible
for the smell?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Methyl salicylate is an excellent Fischer Esterification to do.
The starting reagents smell completely different to the end product (smells like listerine mouth wash) so you have an easy indication if it worked or
not.
I did this version (there are several) :-
https://www.youtube.com/watch?v=lJLP2bcXDqY
Obviously, never stick anything you made in your lab in your mouth.
Ingesting 7g of this will kill you (same as sailicylic acid).
Smelling the mintiness is fine, just not all day long.
[Edited on 8-12-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by ScienceBum  | Ah thanks guys, I appreciate that  Just out of curiosity though, wikipedia says
that there's over 80-something compounds responsible for various fruit smells and flavors and that table only lists one ester per fruit from what I
can see. Is this basically the equivalent of comparing food flavoring additive to the actual food, or are these compounds the "main" ones responsible
for the smell? Just out of curiosity though, wikipedia says
that there's over 80-something compounds responsible for various fruit smells and flavors and that table only lists one ester per fruit from what I
can see. Is this basically the equivalent of comparing food flavoring additive to the actual food, or are these compounds the "main" ones responsible
for the smell? |
If you think about it, human taste buds and olfatory receptors cannot be sensitive to every single chemical (e.g. Nitrogen) and so there is a Limited
range of chemicals they can detect.
The human 'Taste' and 'Smell' range is still enormous, as our detection apparatus combine the levels of the detected chemicals into a mixed signal,
based on the amount of each compound detected.
[Edited on 8-12-2015 by aga]
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
make skatole 
Beginning construction of periodic table display
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not.
Cadverene.
Butyric acid et al ...
You're being capricious diddi.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I just made benzaldehyde recently, and its smell is EXACTLY like almond extract, really lovely stuff.
|
|
|
diddi
National Hazard
   
Posts: 723
Registered: 23-9-2014
Location: Victoria, Australia
Member Is Offline
Mood: Fluorescent
|
|
I though with a name like "ScienceBum", that skatole was appropriate. if I seem capricious, then I had best increase my bipolar meds. I don't want to
be all up and down over Christmas 
Beginning construction of periodic table display
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Amos  | | I just made benzaldehyde recently, and its smell is EXACTLY like almond extract, really lovely stuff. |
There's a reason for that. Essential oil of bitter almond (once treated to remove residual HCN) and it's synthetic equivalent used as almond extract
are pure benzaldehyde.
|
|
|
Acidum
Harmless

Posts: 39
Registered: 2-5-2013
Location: Serbia
Member Is Offline
Mood: Sublimed
|
|
Quote: Originally posted by ScienceBum  | Ah thanks guys, I appreciate that  Just out of curiosity though, wikipedia says
that there's over 80-something compounds responsible for various fruit smells and flavors and that table only lists one ester per fruit from what I
can see. Is this basically the equivalent of comparing food flavoring additive to the actual food, or are these compounds the "main" ones responsible
for the smell? Just out of curiosity though, wikipedia says
that there's over 80-something compounds responsible for various fruit smells and flavors and that table only lists one ester per fruit from what I
can see. Is this basically the equivalent of comparing food flavoring additive to the actual food, or are these compounds the "main" ones responsible
for the smell? |
Mostly, as aga said, we mix several chemical levels (concentrations) into one specific "aroma". Stated esters are just like You said "the main ones",
althought I can recall several compounds that smell like very specific fruits and flowers but that are not present in them.
If you wish, in about two weeks I will be able to dig up through my older scripts from Bioorganic chemistry, and to sniff some sniffable stuff... 
...and then I disappeared in the mist...
|
|
|
Alzador
Harmless

Posts: 19
Registered: 20-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Amos  | | I just made benzaldehyde recently, and its smell is EXACTLY like almond extract, really lovely stuff. |
How did you manage to make benzaldehyde? What method did you use?
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I used potassium chlorochromate, which only oxidizes alcohols to the aldehyde and not a carboxylic acid, to oxidize benzyl alcohol.
Conveniently, there's a YouTube video on the very same oxidation: https://www.youtube.com/watch?v=WaZm8vmPSr4
|
|
|
MrMario
Harmless

Posts: 46
Registered: 2-2-2016
Member Is Offline
Mood: No Mood
|
|
Are molecular sieves (4A) suitable to drive the equilibrium even more to the ester side when making benzoate esters and do they react with H2SO4?
|
|
|